Molecular formula of ethyne
The structural formula of ethyne is? Find the answer to this question and access a vast question bank that is customised for the student. The molecule formula for Ethyne — C 2 H 2.
The molecular formula ethyne is C 2 H 2. From this draw its structural formula and electron - dot structure. The molecular formula of propane is C 3 H 8. From this draw its structural formula. Molecular formula of propane is C 3 H 8. From this, draw its structural formula.
Molecular formula of ethyne
Past Year - 3 Mark Questions. Last updated at May 29, by Teachoo. Molecular formula of Ethane is C 2 H 6. Molecular formula of Ethene is C 2 H 4. Molecular formula of Ethyne is C 2 H 2. Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class. CA Maninder Singh is a Chartered Accountant for the past 13 years and a teacher from the past 17 years. Your browser does not support the audio element. Maths Classes. Old search 1. Old search 2.
Important points to remember:. It is the simplest alkyne that exists in the form of a gas. Each CH molecule produces two hybridized sp orbitals, for a total of four sp hybridized orbitals.
In Chemistry, ethyne is one of the most commonly known examples of the hydrocarbon series called acetylenic series, or alkynes , which has one or more pairs of carbon atoms joined by triple bonds. The common name for ethyne is acetylene. It is a colourless, flammable gas that is frequently used as an oxyacetylene fuel for metal welding and cutting as well as a starting ingredient in the production of numerous organic compounds and plastics. Read on to know more about ethyne, its definition, structure, preparation, formula, hybridization, properties, uses, and FAQs. It is the simplest alkyne that exists in the form of a gas. Pure acetylene is a colourless gas with a pleasant smell.
Simple alkynes are named by the same rules that are used for alkenes see Section 7. They are unsaturated hydrocarbons. Like alkenes have the suffix —ene, alkynes use the ending —yne; this suffix is used when there is only one alkyne in the molecule. Here are the molecular formulas and names of the first ten carbon straight chain alkynes. Number the longest chain starting at the end closest to the triple bond. A 1-alkyne is referred to as a terminal alkyne and alkynes at any other position are called internal alkynes. After numbering the longest chain with the lowest number assigned to the alkyne , label each of the substituents at its corresponding carbon. While writing out the name of the molecule, arrange the substituents in alphabetical order. If there are more than one of the same substituent use the prefixes di, tri, and tetra for two, three, and four substituents respectively. These prefixes are not taken into account in the alphabetical order.
Molecular formula of ethyne
C 2 H 2 is the simplest alkyne chemical compound with the chemical name Acetylene. Acetylene is also called Ethyne or Narcylen or Vinylene. It is widely used as a chemical building block and as a fuel. In its pure form, it is unstable and is handled as a solution. It is an unsaturated compound the two carbon atoms in it are linked together with a double bond. Vinylene is a colourless gas which has a mild ether-like odour. It is easily soluble in water, chloroform, acetone, and benzene. It is slightly soluble in carbon disulfide and ethanol. It is lighter when compared to air and easily ignites.
Bay gardens obituary
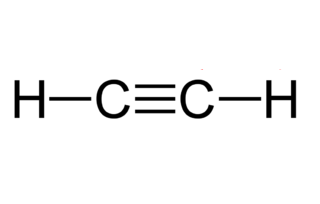
The ethyne molecule has a linear molecular form. Draw the electron dot structure of ethyne and also draw its structure Displaying ads are our only source of revenue. Ethyne FAQs What are the common names for ethyne? In Indian rupees, 1 trillion is equal to how many crores? Trending Topics. Ethyne Structure In ethyne, the two carbon atoms are linked together with the help of a triple bond, and the hydrogen atoms are connected to each carbon atom via a single covalent bond. In this structure, the carbon atom will have two half-filled 2p orbitals. The chemical equation for the reaction of calcium carbide with water is shown below. Download NEET question paper. What is zone refining and what is its significance in manufacturing transistors?
Ethyne, also known as acetylene, is an organic chemical compound with the chemical formula C 2 H 2. Since the entire chemical composition only features hydrogen and carbon atoms, this compound is a hydrocarbon.
Draw electron-dot and line structures for ammonia molecule. The molecular formula of sulphur is S 8 in which eight sullphur atoms Examples include acetylides of sodium, copper, and silver. The boiling point is also the sublimation point for the gas. It's free :. Ethyne is proven to exist as a colourless gas with no discernible smell under standard pressure and temperature settings. What is zone refining and what is its significance in manufacturing transistors? These two pairs of p orbitals produce two pi bonds instead of participating in the hybridisation, resulting in the development of a triple bond. Another significant usage of acetylene, especially in China, is the manufacturing of derivatives of acrylic acid. Ethyne has a molecular mass of This reaction is still used extensively for the production of ethyne at the commercial level. Find the answer to this question and access a vast question bank that is customised for the student. Write the structural formula of butane.. Each CH molecule produces two hybridized sp orbitals, for a total of four sp hybridized orbitals.

Remarkable topic