Molar weight of nitrogen
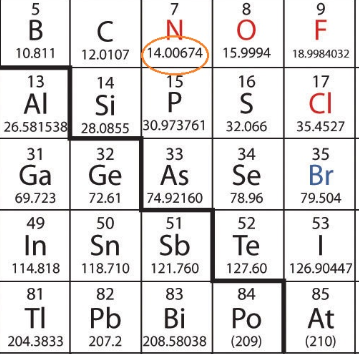
Nitrogen is a chemical element ; it has symbol N and atomic number 7. Nitrogen is molar weight of nitrogen nonmetal and the lightest member of group 15 of the periodic tableoften called the pnictogens. It is a common element in the universeestimated at seventh in total abundance in the Milky Way and the Solar System.
As we described in Section 4. The number of things in a mole is large, very large 6. We are all familiar with common copy-machine paper that comes in sheet reams. If you stacked up 6. The mole is a huge number, and by appreciating this, you can also gain an understanding of how small molecules and atoms really are. Chemists work simultaneously on the level of individual atoms, and on the level of samples large enough to work with in the laboratory.
Molar weight of nitrogen
The molecular weight of a substance, also called the molar mass , M, is the mass of 1 mole of that substance, given in M gram. Molecular weight is represented by the same number in all unit systems regardless of the system used. For this reason, in many cases the unit for the molecular weight is not mentioned; however, one must realize that it is not a dimensionless parameter. The molecular weight of a pure compound is determined from its chemical formula and the atomic weights of its elements. Example: The molecular weight of ethanol C 2 H 5 OH To calculate the molecular weight of ethanol, the molecular weight of each atom in the molecule is summed:. See also Physical data for hydrocarbons , Physical data for alcohols and carboxylic acids , Physical data for organic nitrogen compounds and Physical data for organic sulfur compounds. Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with older versions of the amazing SketchUp Make and the newer "up to date" SketchUp Pro. Translate this page to Your Own Language. If you want to promote your products or services in the Engineering ToolBox - please use Google Adwords. Temperature o C K o F.
BBC News.
.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Molar weight of nitrogen
Nitrogen is a chemical element ; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table , often called the pnictogens. It is a common element in the universe , estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure , two atoms of the element bond to form N 2 , a colorless and odorless diatomic gas. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. Elemental nitrogen is usually produced from air by pressure swing adsorption technology. Many industrially important compounds, such as ammonia , nitric acid, organic nitrates propellants and explosives , and cyanides , contain nitrogen.
Villa porno
Nitrogen can be used as a replacement, or in combination with, carbon dioxide to pressurise kegs of some beers , particularly stouts and British ales , due to the smaller bubbles it produces, which makes the dispensed beer smoother and headier. It also results in very large electrostatic forces of attraction between the nucleus and the valence electrons in the 2s and 2p shells, resulting in very high electronegativities. Nitrogen occurs in all organisms, primarily in amino acids and thus proteins , in the nucleic acids DNA and RNA and in the energy transfer molecule adenosine triphosphate. They were well-known by the Middle Ages. Bibcode : JChEd.. The 2p subshell is very small and has a very similar radius to the 2s shell, facilitating orbital hybridisation. Many industrially important compounds, such as ammonia , nitric acid, organic nitrates propellants and explosives , and cyanides , contain nitrogen. They hydrolyse only very slowly to give ammonia or nitrogen. Like carbon, nitrogen tends to form ionic or metallic compounds with metals. Nitrosyl chloride NOCl behaves in much the same way and has often been used as an ionising solvent. Elsevier Academic Press. In other projects. They are normally prepared by three methods: [26]. Nitrogen trichloride NCl 3 is a dense, volatile, and explosive liquid whose physical properties are similar to those of carbon tetrachloride , although one difference is that NCl 3 is easily hydrolysed by water while CCl 4 is not.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber.
In concentrated sulfuric acid, nitric acid is protonated to form nitronium , which can act as an electrophile for aromatic nitration: [68]. Retrieved 27 September Privacy Policy We don't collect information from our users. Retrieved The latter two compounds are somewhat difficult to study individually because of the equilibrium between them, although sometimes dinitrogen tetroxide can react by heterolytic fission to nitrosonium and nitrate in a medium with high dielectric constant. When the ammonia is taken up by plants, it is used to synthesise proteins. Like carbon, nitrogen tends to form ionic or metallic compounds with metals. Journal of Agricultural and Food Chemistry. It is a violent oxidising agent. Download as PDF Printable version. The risks posed by them". Both compounds may be easily prepared by decomposing a dry metal nitrate. Archived from the original on June 23, Nevertheless, the analogy is not exact due to the ease of nucleophilic attack at boron due to its deficiency in electrons, which is not possible in a wholly carbon-containing ring. It is used in the cryopreservation of biological materials such as blood and reproductive cells sperm and eggs.

0 thoughts on “Molar weight of nitrogen”