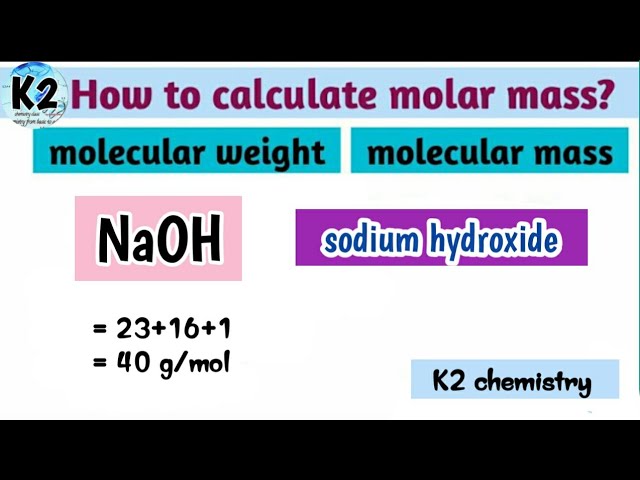
Molar mass of sodium hydroxide
Molar mass of NaOH Sodium hydroxide is Then, molar mass of sodium hydroxide, lookup atomic weights for each element in periodic table : Na: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students.
Sodium hydroxide , also known as lye and caustic soda , [1] [2] is an inorganic compound with the formula NaOH. Sodium hydroxide is a highly corrosive base and alkali that decomposes lipids and proteins at ambient temperatures and may cause severe chemical burns. It is highly soluble in water , and readily absorbs moisture and carbon dioxide from the air. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the making of wood pulp and paper , textiles , drinking water , soaps and detergents , and as a drain cleaner.
Molar mass of sodium hydroxide
.
Tm OH 3. This helps homogenise cement mixes, preventing segregation of sands and cement, decreases the amount of water required in a mix and increases workability of the cement product, be it mortar, render or concrete. Unit converters.
.
This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration or recalculating grams per ml to moles. You can also calculate the mass of a substance needed to achieve a desired molarity. This article will provide you with the molarity definition and the molarity formula. To understand the topic as a whole, you will want to learn the mole definition, read a paragraph about the molarity units, as well as read a comparison of two misleading concepts: molarity formula vs molality formula. What is more, we prepared for you some interesting examples of molar solutions and a short step-by-step tutorial on how to calculate the molarity of a concentrated solution.
Molar mass of sodium hydroxide
As we described in Section 4. The number of things in a mole is large, very large 6. We are all familiar with common copy-machine paper that comes in sheet reams. If you stacked up 6. The mole is a huge number, and by appreciating this, you can also gain an understanding of how small molecules and atoms really are. Chemists work simultaneously on the level of individual atoms, and on the level of samples large enough to work with in the laboratory. The concept that allows us to bridge these two scales is molar mass. Molar mass is defined as the mass in grams of one mole of a substance. The atomic mass of an element is the relative average of all of the naturally occurring isotopes of that element and atomic mass is the number that appears in the periodic table.
Height cambridge dictionary
This helps homogenise cement mixes, preventing segregation of sands and cement, decreases the amount of water required in a mix and increases workability of the cement product, be it mortar, render or concrete. Find atomic masses: look up the atomic masses of each element present in the compound. For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. Chemical compound with formula NaOH. Unlike sodium hydroxide, which is soluble, the hydroxides of most transition metals are insoluble, and therefore sodium hydroxide can be used to precipitate transition metal hydroxides. It also produces heat when reacted with acids. Ba OH 2. IDLH Immediate danger. LD 50 median dose. In other projects. Ac OH 3. Sodium hydroxide is a popular strong base used in industry. For example, in the petroleum industry, sodium hydroxide is used as an additive in drilling mud to increase alkalinity in bentonite mud systems, to increase the mud viscosity , and to neutralize any acid gas such as hydrogen sulfide and carbon dioxide which may be encountered in the geological formation as drilling progresses. Sodium hydroxide is also produced by combining pure sodium metal with water.
You can go from moles of sodium hydroxide, "NaOH" , to grams of sodium hydroxide by using a conversion factor called molar mass. The molar mass tells you the mass of exactly one mole of a given substance.
The atoms are arranged in a hydrargillite -like layer structure, with each sodium atom surrounded by six oxygen atoms, three each from hydroxide ions and three from water molecules. Sodium hydroxide is used in the manufacture of sodium salts and detergents, pH regulation, and organic synthesis. Lattice constant. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Despite solubility in propylene glycol it is unlikely to replace water in saponification due to propylene glycol's primary reaction with fat before reaction between sodium hydroxide and fat. Ac OH 3. Touching a sodium hydroxide solution with bare hands, while not recommended, produces a slippery feeling. This helps homogenise cement mixes, preventing segregation of sands and cement, decreases the amount of water required in a mix and increases workability of the cement product, be it mortar, render or concrete. Historically, sodium hydroxide was produced by treating sodium carbonate with calcium hydroxide in a metathesis reaction which takes advantage of the fact that sodium hydroxide is soluble, while calcium carbonate is not. Retrieved June 12, BBC Newsnight. One mole contains exactly 6.

Interestingly :)
I consider, that you are not right. I am assured. I can prove it. Write to me in PM, we will talk.