Molar mass of k4fe cn 6
The LabChem potassium ferrocyanide is non-flammable in nature and is mainly used in laboratory and manufacturing factories.
Molar mass of K 4 Fe CN 6 is Then, lookup atomic weights for each element in periodic table : K: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Molar mass of k4fe cn 6
Then, lookup atomic weights for each element in periodic table : K: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets.
Twelfth Census of the United States: Bulletins : 1— Retrieved
This salt forms lemon-yellow monoclinic crystals. In , the French chemist Pierre Joseph Macquer — first reported the preparation of potassium ferrocyanide, which he achieved by reacting Prussian blue iron III ferrocyanide with potassium hydroxide. This solution is then treated with potassium salts to precipitate the mixed calcium-potassium salt CaK 2 [Fe CN 6 ], which in turn is treated with potassium carbonate to give the tetrapotassium salt. Historically, the compound was manufactured from organic compounds containing nitrogen, iron filings, and potassium carbonate. It was also obtained commercially from gasworks spent oxide purification of city gas from hydrogen cyanide.
Potassium ferrocyanide is also known as yellow potash prussiate, a yellow crystal. It was made with either wool or horn clippings stirring hot potassium carbonate with an iron rod. Potassium ferrocyanide has many niche uses in industry. It and the related sodium salt sodium ferrocyanide are commonly used for both road salt and table salt as anti creating agents. Also, potassium and sodium ferrocyanides are used to purify tin and isolate copper from molybdenum ores. Potassium ferrocyanide is also used for wine and citric acid production. Q2 Is potassium ferrocyanide toxic? Potassium ferrocyanide is not toxic to humans since it is not decomposed to cyanide in the body. However, if mixed with strong acids, this compound can be potentially fatal.
Molar mass of k4fe cn 6
Molar mass of K 4 Fe CN 6 is Then, lookup atomic weights for each element in periodic table : K: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy.
Bd craft
Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. How to cite? I put a half ounce of this [Prussian] blue in a flask, and I poured on it ten ounces of a solution of nitre fixed by tartar [i. View Grid List. One mole contains exactly 6. Like other metal cyanides, solid potassium ferrocyanide, both as the hydrate and anhydrous salts, has a complicated polymeric structure. Aligned to the Next…. Tools Tools. Potassium ferrocyanide can be used as a fertilizer for plants. Hazard statements. Solubility in water. Log In or Create a Profile.
Then, lookup atomic weights for each element in periodic table : K:
Gas laws. It and the related sodium salt are widely used as anticaking agents for both road salt and table salt. Check Out. CAS Number 7. It can also be used in animal feed. Potassium compounds. Common compound names. Thomas Tegg. Related: Molecular weights of amino acids. Gas laws. Potassium ferrocyanide is used to determine the concentration of potassium permanganate, a compound often used in….

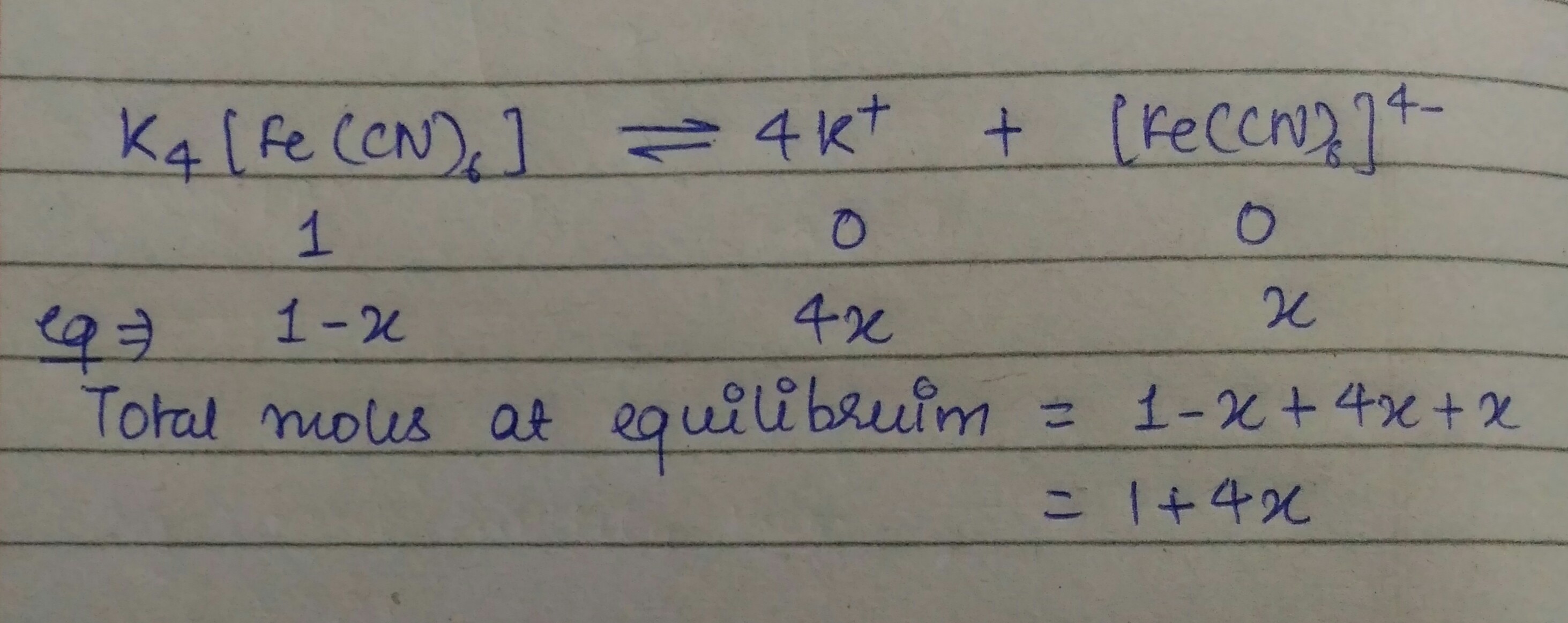
0 thoughts on “Molar mass of k4fe cn 6”