Mgcl2 is ionic or covalent
Magnesium chloride is an ionic compound while hydrogen chloride is a covalent compound.
Since you have started studying chemistry, you are aware of both of these chemical elements called magnesium and chlorine. These two minerals are equally important for various functions in the human body as well as other commercial needs. Magnesium or Mg and chlorine or Cl combine to form magnesium chloride. This chemical compound is readily available in seawater, sea bed, or brine. This mineral can also be extracted by the process of solution mining and evaporation of seawater.
Mgcl2 is ionic or covalent
.
Thus, the ratio of magnesium ion: chloride ion is 1: 2 and the simplest magnesium chloride chemical formula is MgCl 2. This is because of the presence of chlorine in MgCl 2, and an excess amount of chlorine can be toxic for plants.
.
MgCl 2 can be extracted from seawater or brine and is chemically named Magnesium Chloride. Chloromagnesite is non-combustible but when heated liberates irritating toxic gas or fumes. Inhaling this compound causes severe coughing. Well water consists of dissolved magnesium chloride. It was used in locomotive boilers located on the Trans-Australian Railway and resulted in serious and costly maintenance problems. This problem occurred during the steam era. There was no facility for freshwater watercourses, therefore they had to rely on bore water. The locomotive boilers could last for less than a quarter. The maintenance cost was expensive.
Mgcl2 is ionic or covalent
MgCl2 is an ionic halide salt consisting of magnesium and chlorine elements. It is inorganic in nature that may appear as a white or colorless crystalline solid compound. In its anhydrous form, it consists of about We can extract hydrated MgCl2 from brine. Also, we can witness varying degrees of bitterness in MgCl2 solutions which in turn depends on the percentage of Mg present in them.
Excerpt synonym
Places that are typically struck by extreme snowstorms use magnesium chloride to treat the icy conditions of various transport ways. On rapid heating, the melting point of the Magnesium Chloride is degrees Celsius which is degrees Fahrenheit. Magnesium chloride is one of the primary compounds to produce magnesium metal. This way, anhydrous MgCl 2 is extracted from its hydrous state. There are a few more chemical names by which Magnesium Chloride is referred, such as Magnesium Dichloride, Chloromagnesite, and Magnesium ii chloride. Thus Magnesium has two electrons in its outermost orbit or it can be said that Magnesium has 2 valence electrons. This reaction is accomplished by electrolysis. Moreover, by joining our online doubt-clearing classes, you can prepare chemistry at its best level. Due to various usages for different industrial and commercial purposes, this ionic salt is produced in large amounts. Which of the following compounds are ionic and which are covalent? Its water solubility for anhydrous is as under:. So, magnesium ions form the ionic bond with two chlorine ions. For anhydrous Magnesium Chloride Formula. For hexahydrate gram per ml water at 20 o C.
In ordinary chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements.
South America. Since you have started studying chemistry, you are aware of both of these chemical elements called magnesium and chlorine. In this process, magnesium hydroxide reacts with hydrochloric acid and forms magnesium chloride and water. This ionic bond helps to complete the electron transferring process between atoms. Ans: b. Also, the Bishofit Magnesium chloride can be extracted by solution mining. Its boiling point is degrees Celsius, which in Fahrenheit becomes degrees. From the below content, you can learn the structure, property, preparation, and usages of this compound. Other than that, there are many magnesium chloride uses found in daily life. Magnesium Chloride is just as important a topic for the chemistry students as its components, Magnesium, and Chlorine.

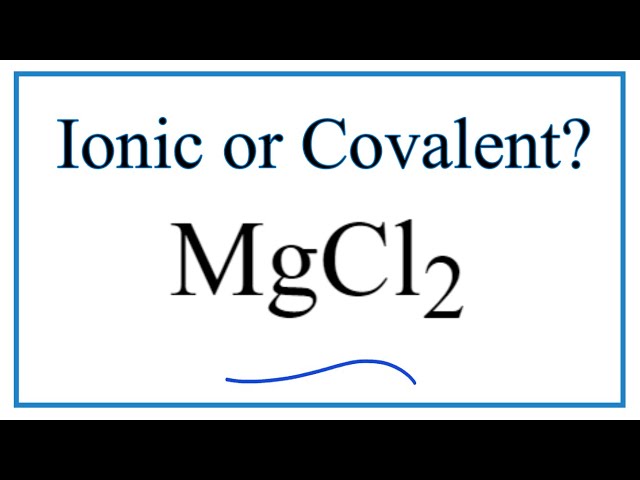
0 thoughts on “Mgcl2 is ionic or covalent”