Meq to ml
Wiki User.
The science world is filled with different ways to express the vital concept of concentration , which is the amount of something present per unit volume. This "amount" often has units of mass but can include virtually anything that can be quantified: gas particles, photons and more. The volume in question is often a solution , which involves a substance called a solute in this context dissolved in a liquid called a solvent. When solid dissolves in a solvent to create a solution, the concentration of the solution can be expressed in a variety of ways. This relates to the fact that chemicals react with each other not on the basis of mass but on the basis of the ratio of individual "pieces," regardless of size. The concept of moles and equivalents, and thus millimoles and milliequivalents , underlies this relationship, and it is of vital importance in medicine and clinical pharmacology. In an example of a simple chemical reaction, one atom of potassium K can react with one atom of chlorine Cl to form a molecule of potassium chloride KCl with nothing left over.
Meq to ml
.
How to Calculate Mass in Grams of a Molecule. How many milliliter is equal to 1, meq to ml. The volume in question is often a solutionwhich involves a substance called a solute in this context dissolved in a liquid called a solvent.
.
Fast , Accurate Calculations at Your Fingertips! The milliequivalents calculator calculates the milliequivalents of a compound given the weight in milligrams, the molecular weight and valence. Enter the weight of the substance in milligrams, the molecular weight and the valence into the calculate to determine the milliequivalents mEq. Milliequivalents mEq are a chemical unit that measures the chemical combining activity of an electrolyte. The milliequivalent expresses the concentration of electrolytes in solution and calculated as the product of the millimoles mmol and the valence of the electrolyte. The milliequivalents mEq can be calculated using the following formula:. To calculate milliequivalents, multiply the milligrams of the substance by its valence and then divide by its molecular weight. Watch the video below where I demonstrate how to calculate milliequivalents if you want a more detailed explanation. To better understand how milliequivalents mEq calculations work, let's consider an example problem.
Meq to ml
The science world is filled with different ways to express the vital concept of concentration , which is the amount of something present per unit volume. This "amount" often has units of mass but can include virtually anything that can be quantified: gas particles, photons and more. The volume in question is often a solution , which involves a substance called a solute in this context dissolved in a liquid called a solvent. When solid dissolves in a solvent to create a solution, the concentration of the solution can be expressed in a variety of ways. This relates to the fact that chemicals react with each other not on the basis of mass but on the basis of the ratio of individual "pieces," regardless of size. The concept of moles and equivalents, and thus millimoles and milliequivalents , underlies this relationship, and it is of vital importance in medicine and clinical pharmacology.
Graco 4ever 4-in-1 car seat
Kevin Beck holds a bachelor's degree in physics with minors in math and chemistry from the University of Vermont. A solution contains 30 mg of NaCl table salt per mL of solution. How to Dissolve Magnesium Chloride. Meq is a measure of charge. Still have questions? Formerly with ScienceBlogs. How many liters is there in a milliliter? Note: the molecular weight of NaCl is This "amount" often has units of mass but can include virtually anything that can be quantified: gas particles, photons and more. How to Calculate Millimolars.
Then, this last equation is converted to milliequivalents mEq by dividing both sides by , which yields the equation above. As a shortcut, then, the equation can be used remembering that in this instance, the unit of milligram is affixed to the molecular weight. Of note is that the molecular or atomic weights are usually assigned the unit of gram; however, for milliequivalents we assign the unit of milligram.
How to Calculate Millimolars. All Rights Reserved. How many grams in 20 mEq of Potassium? Formerly with ScienceBlogs. Resources Leaderboard All Tags Unanswered. How many mm equal 1 meq? This means that KCl has a valence of 2. The concept of moles and equivalents, and thus millimoles and milliequivalents , underlies this relationship, and it is of vital importance in medicine and clinical pharmacology. Best Answer. Potassium chloride extended-release capsules, USP, 10 mEq is an oral dosage form of microencapsulated potassium chloride containing mg of potassium chloride USP equivalent to 10 mEq of potassium. Wiki User. This equation assumes that both mass and MW, or molecular weight the same as molar mass but applied to molecules instead of single atoms , are given in milligrams.

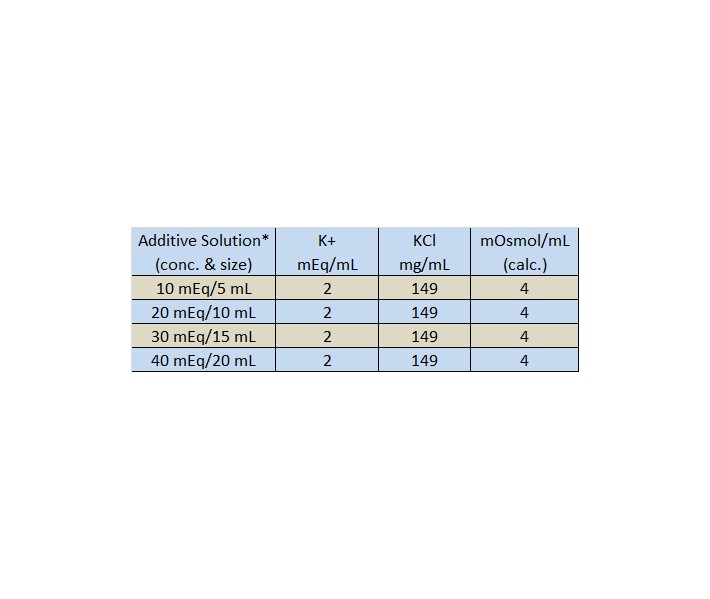
All can be