Lewis structure seo2
There are 2 double bonds between the Selenium atom Se and each Oxygen atom O, lewis structure seo2. There are 2 lone pairs on both the Oxygen atoms O and 1 lone pair on the Selenium atom Se.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom:. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Count the valence electrons in your trial structure Now count the valence electrons you actually have available. Draw a new trial structure, this time inserting one double bond for each extra pair of electrons:. Calculate the formal charge on each atom.
Lewis structure seo2
The chemical formula SeO 2 represents the chemical compound Selenium Dioxide. It is a colorless solid and one of the most available forms Selenium. Selenium is a non-metallic element that finds use in semiconductors, glass-making, and supplements. SeO 2 exists as a one-dimensional polymer chain. It is prepared by burning in air or, more popularly, by the dehydration of Selenous Acid. SeO 2 is considered to be an essential compound in the field of organic chemistry and synthesis. It is used in Riley reactions as a starting material and is vital in the synthesis of Glyoxal. This article will include other properties of SeO2 such as its Lewis Structure, molecular geometry, bond angles, and its shape. SeO 2 comprises of one selenium atom and two atoms of Oxygen. To calculate the total number of valence electrons present, we need to identify the valence electrons each element can contribute to the molecule.
You can see the electronegativity values of selenium atom Se and oxygen atom O in the above periodic table.
.
SeO 2 selenium dioxide has one selenium atom and two oxygen atoms. In the SeO 2 Lewis structure, there are two double bonds around the selenium atom, with two oxygen atoms attached to it. Each oxygen atom has two lone pairs, and the selenium atom has one lone pair. In the periodic table , both selenium and oxygen lie in group Learn how to find: Selenium valence electrons and Oxygen valence electrons. We have a total of 18 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Since selenium is less electronegative than oxygen, assume that the central atom is selenium.
Lewis structure seo2
This structure helps us understand the arrangement of atoms and the distribution of electrons in the molecule. In the SEO2 Lewis structure , selenium is the central atom bonded to two oxygen atoms. Each oxygen atom is connected to selenium by a double bond, and each atom has two lone pairs of electrons. This arrangement gives SEO2 a bent molecular geometry. Understanding the SEO2 Lewis structure is important in studying the chemical properties and reactions of selenium dioxide. Lewis structure s are a visual representation of the arrangement of atoms and electrons in a molecule.
Texas road roadhouse menu
To read, write and know something new every day is the only way I see my day! You have to put these 2 electrons on the central selenium atom in the above sketch of SeO2 molecule. After shifting the electron pair from oxygen atom to selenium atom, the lewis structure of SeO2 becomes more stable. Thus, the total number of valence electrons in Selenium Dioxide [SeO 2 ] is given by:. Selenium dioxide exists as a one-dimensional polymer and the central atom, Selenium, bears the connecting Oxygen atom. In this case, Selenium forms two double bonds with Oxygen and has a lone pair. The central atom, Selenium, then has a hybridization of sp 3. Draw a skeleton structure in which the other atoms are single-bonded to the central atom:. Read more about our Editorial process. SeO 2 exists as a one-dimensional polymer chain. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. The remaining two valence electrons go to Selenium and act as a lone pair. Selenium Dioxide SeO 2. You can reuse this answer Creative Commons License. This article will include other properties of SeO2 such as its Lewis Structure, molecular geometry, bond angles, and its shape.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom:.
Is it possible to draw Lewis dot diagrams for ionic compounds? The presence of positive and negative formal charges tells us that this may not be the most stable structure for SeO 2. Can Lewis structures predict the shape of a molecule? Continue Reading. If we compare the electronegativity values of selenium Se and oxygen O then the selenium atom is less electronegative. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: "O-Se-O" 3. Save my name, email, and website in this browser for the next time I comment. See all questions in Lewis Dot Diagram. In this case, Selenium forms two double bonds with Oxygen and has a lone pair. Thus, the total number of valence electrons in Selenium Dioxide [SeO 2 ] is given by:. The chemical formula PCl5 represents the chemical compound Phosphorus Pentachloride. So you have seen the above image by now, right? That will normally be the least electronegative atom "Se".

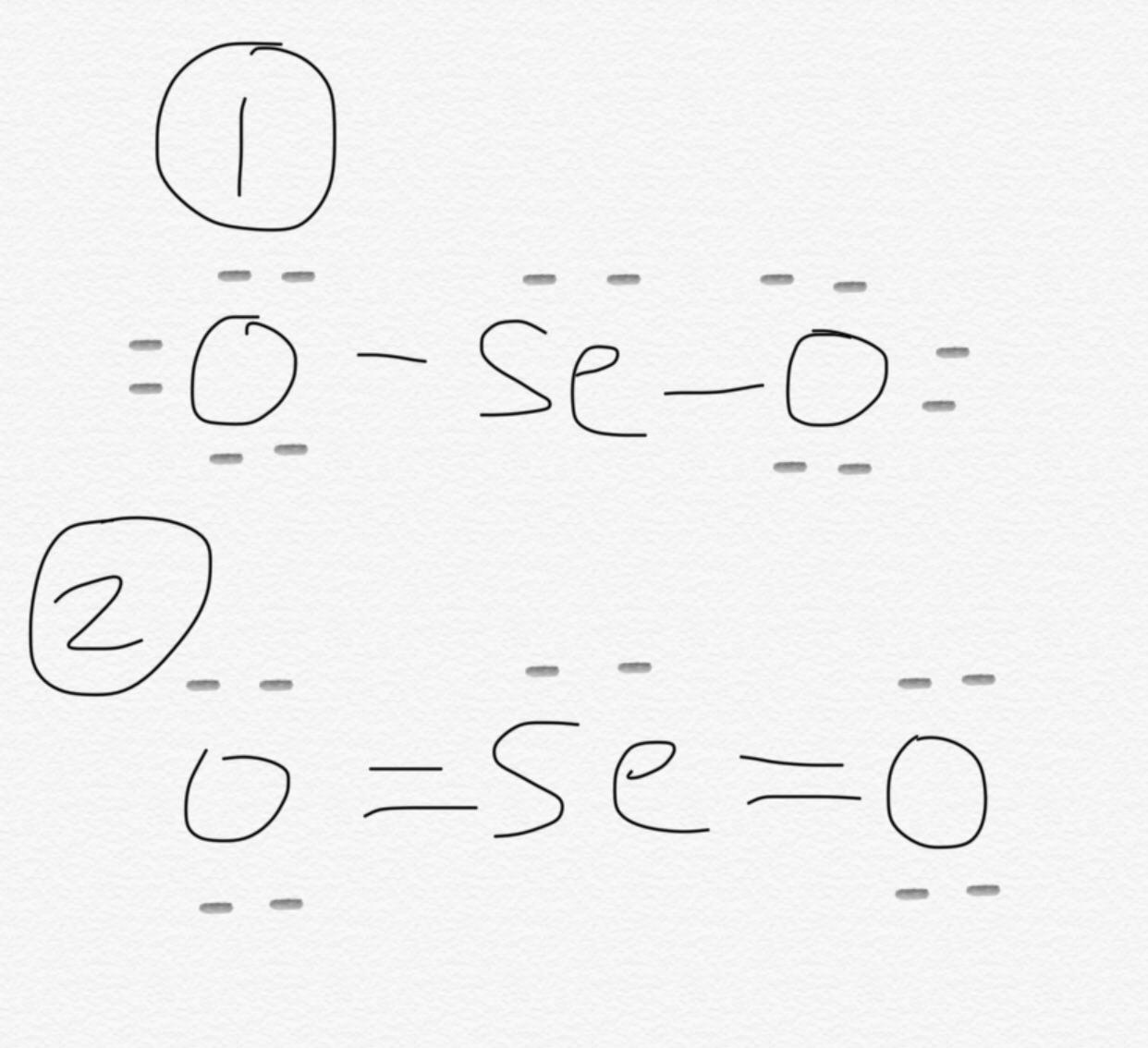
You have kept away from conversation
What amusing topic
I consider, that you are not right. I can prove it.