Lewis structure of sf6
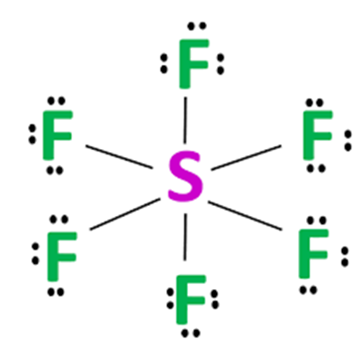
Sulfur atom S is the central atom, fluorine atom F is the external atom, sulfur atom S and each fluorine atom F are connected by a single bond, each lewis structure of sf6 atom F has three lone pairs of electrons, and the central atom is symmetrically distributed around.
This article is about the SF6 Lewis Structure, the Molecular geometry, and the formal charge present in the molecule. A Lewis Structure is a graphical representation of the valence shell electrons of a molecule. The Lewis structure was initially proposed by famous scientist Gilbert N. The Lewis structure is important in chemistry because it can predict the number of bonds, nonbonding electrons, and bonding electron structure. Lewis structure does not try to explain the molecular shape, bond formation, or electron sharing between atoms. It is the most basic and limiting explanation of the electrical structure.
Lewis structure of sf6
Draw the Lewis structure of HCN. Draw the Lewis structure of B e C l 2. Draw the Lewis structure of C l O 4 per chlorate ion. Write the Lewis dot structure of C O molecule. Draw the Lewis structure of nitric acid, H N O 3. Draw the Lewis structure for SF6. Which one of the following molecules contains no pi - bond? Which of the following is a polar moleule? Which of the following is paramagnetic? According to MO theory which of thhe following lists makes the nitroge In the case of alkali metals, the covalent character decreases in the The state of hybridization of C2, C3, C5, and C6 of the hydrocarbon Arrange the following ions in the order of decreasing X-O bond length
Because there are 6 sigma bonds surrounding the Sulphur atom, the valence shell of Sulphur has 12 electrons.
.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions.
Lewis structure of sf6
SF6 or sulfur hexafluoride is an inorganic and one of the most stable gases that are known in chemistry. This gas has more density than air. There is also no taste of the gas as such. SF6 is noncombustible and nonflammable in nature. However, under extreme heat and pressure, it might burst out of its storage container and rocket into the air. SF6 can react with a few compounds to further disassociate and take part in the following reactions.
Doctors on broadway reservoir
What is Sulfur Hexafluoride SF6? To draw a Lewis structure of a molecule, there are a few general steps to follow. As a result, SF6 is non-polar. The central atom must have a high valence or minimal electronegativity. As a result, arrange it in the middle and all Fluorine atoms around it. When many atoms in an ion or molecule are positively or negatively charged, or when there are more charges on the atoms e. Chemical and physical properties of Sulfur hexafluoride Dec 21, Sulfur hexafluoride is an organic, colorless, odorless, noninflammable, nontoxic, and long-lived atmospheric lifetime of — years gas. To draw lone pairs, it is important to remember how many total numbers of electron pairs are present. Mar 6, Because Sulphur is less electronegative than Fluorine, it will assume the middle position.
Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas.
Lewis structure of any molecule is important because. Note that the central S atom has a total of 12 valence electrons around it in the skeleton structure. To draw lone pairs, it is important to remember how many total numbers of electron pairs are present. Sulfur atom S is the central atom, fluorine atom F is the external atom, sulfur atom S and each fluorine atom F are connected by a single bond, each fluorine atom F has three lone pairs of electrons, and the central atom is symmetrically distributed around. To get the total number of electron pairs, divide the total number of valence electrons by two. The enolic form of butanone contains Density liquid. The state of hybridization of C2, C3, C5, and C6 of the hydrocarbon Draw the Lewis structure of nitric acid, H N O 3. This understanding will eventually allow us to identify molecule forms and chemical characteristics. Mar 6, Is lemonade acidic or a base? Choosing the Center Atom.

Interesting variant
The properties turns out, what that
Very amusing opinion