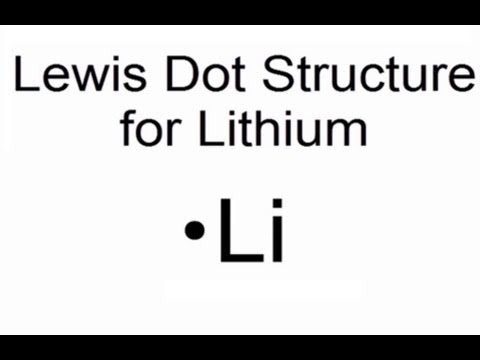
Lewis structure for lithium
A dot is used to represent a valence electron. Valence electrons occupy the highest energy level also known as the valence shell. The chemical symbol of an element is surrounded by a number of dots.
Lewis symbol of element shows the symbol of element with valence electrons shown as dots placed on top, bottom, left, and right sides of the symbol. Valence electrons up to four are shown as a single dot on either side of the symbol. The 5th, 6th, 7th, and 8th valence electron dots are paired with any of the first four dots. For example, represent hydrogen, beryllium, boron, carbon, nitrogen, fluorine, and neon with 1, 2, 3, 4, 5, 6, 7, and 10 valence electrons, respectively. Lewis symbols of first twenty elements are shown in section 2.
Lewis structure for lithium
Lewis symbols use dots to visually represent the valence electrons of an atom. Lewis symbols also known as Lewis dot diagrams or electron dot diagrams are diagrams that represent the valence electrons of an atom. Lewis structures also known as Lewis dot structures or electron dot structures are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds. An atom consists of a positively charged nucleus and negatively charged electrons. Careful investigations have shown that not all electrons within an atom have the same average position or energy. As an example, a neutral atom of gold Au contains 79 protons in its nucleus and 79 electrons. The first principal energy level, which is the one closest to the nucleus, can hold a maximum of two electrons. The second principal energy level can have 8, the third can have 18, and so on, until all 79 electrons have been distributed. The outermost principal energy level is of great interest in chemistry because the electrons it holds are the furthest away from the nucleus, and therefore are the ones most loosely held by its attractive force; the larger the distance between two charged objects, the smaller the force they exert on each other.
When counting electrons, negative ions should have extra electrons placed in their Lewis structures; positive ions should have fewer electrons than an uncharged molecule. A dot is used to represent a valence electron.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram A representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used.
Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in the molecule. Lewis structures can also be useful in predicting molecular geometry in conjuntion with hybrid orbitals. A compound may have multiple resonance forms that are also all correct Lewis structures. This section will discuss the rules for writing out Lewis structures correctly. Writing out Lewis structures can be at times, tricky and somewhat difficult. A compound can have multiple Lewis Structures that contribute to the shape of the overall compound, so one Lewis structure of a compound may not necessarily be exactly what the compound looks like. But before we begin, there are a few things to know.
Lewis structure for lithium
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. The order in which the positions are used does not matter.
Winx dolls
The octet rule states that atoms have a tendency to complete the octet of electrons, that is, to achieve a Noble Gas Group 18 electron configuration. Each nitrogen atom Group 15 has 5 valence electrons A nitrogen atom needs 3 more electrons in order to complete its valence shell, that is, to make up 8 electrons in the L shell. Note that the valence shell of Period 1 and Period 2 elements cannot be expanded because d orbitals are not available in the first and second energy levels. Resonance theory or Molecular Orbital MO theory give more satisfactory descriptions in this instance. In that case, the transition metal groups are included in the counting and the groups indicated at the top of the periodic table have numbers 1, 2, 13, 14, 15, 16, 17, The shared pair of dots represents a pair of bonding electrons, a covalent bond. Search site Search Search. Fluorine and neon have seven and eight dots, respectively:. Hydrogen will share its electron with fluorine to form a bonding pair of electrons covalent bond so that the hydrogen atom has a share in 2 valence electrons electronic configuration of helium and fluorine has a share in 8 valence electrons electronic configuration of neon. Footnotes 1. Group 18 Noble Gas elements will not form ions. Achieving an octet of valence electrons: Two fluorine atoms are able to share an electron pair, which becomes a covalent bond. Lithium which has 3 electrons in total, has 2 electrons in the first energy level K shell just like helium, but these two electrons are not available for bonding so they are not valence electrons. Do you understand this? Lewis Structure electron dot diagram for the nitrogen molecule, N 2 , :N..
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule.
If yes, the Lewis structure is complete, e. Chemical reactivity of all of the different elements in the periodic table depends on the number of electrons in that last, outermost level, called the valence level or valence shell. The next atom, lithium, has an electron configuration of 1 s 2 2 s 1 , so it has only one electron in its valence shell. Fluorine atom Group 17 has 7 valence electrons Fluorine atom needs one more electron to complete its valence shell, that is, to make 8 electrons in the L shell. An example is a reaction between hydrogen having one valence electron and carbon having four valence electrons react to form CH 4 molecule. For example, PCl 3 has octet complete, but PCl 5 has 10 valence electrons. Answers The first two electrons in a valence shell are s electrons, which are paired. Because the side is not important, the Lewis electron dot diagram could also be drawn as follows:. Helium He , at the very top of this column is an exception because it has two valence electrons; its valence level is the first principal energy level which can only have two electrons, so it has the maximum number of electrons in its valence level as well. Each O atom starts out with six red electrons and C with four black electrons, and each bond behind an O atom and the C atom consists of two electrons from the O and two of the four electrons from the C. When counting electrons, negative ions should have extra electrons placed in their Lewis structures; positive ions should have fewer electrons than an uncharged molecule. Lewis symbols for the elements depict the number of valence electrons as dots. The term hydron therefore refers to positively charged ions of the naturally occuring isotopic mix of hydrogen atoms. The table below shows the Lewis Structures for elements with atomic numbers 3 to 10 in the periodic table:. Next Section.

Yes you are talented