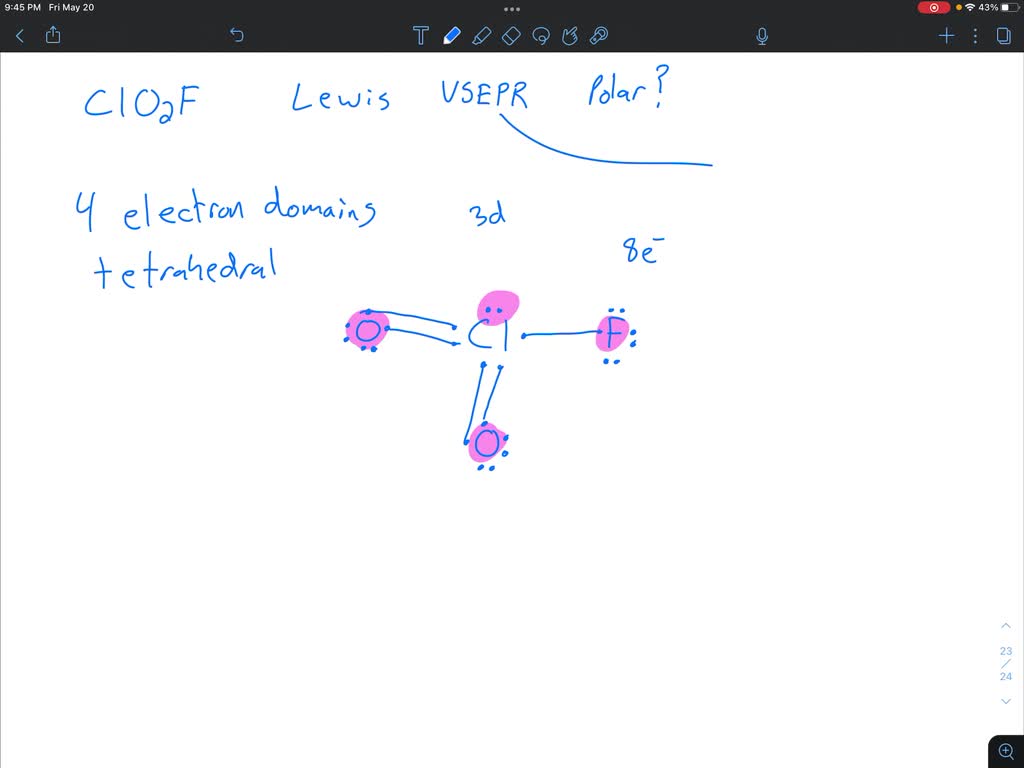
Lewis structure for clo2f
Molar mass of ClO 2 F Chloryl fluoride is Then, lookup atomic weights for each element in periodic table : Cl:
Q: A Lewis structure with placeholder elements is shown below. If the formal charge of the central atom…. A: In the given Lewis structure, the central atom must have 5 valence electron which is possible for…. Q: A student proposes the following Lewis structure for the isocyanate NCo ion. Q: Draw the Lewis structure for in SO
Lewis structure for clo2f
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The Chloryl fluoride molecule contains a total of 3 bond s. There are 3 non-H bond s , 2 multiple bond s , and 2 double bond s. Images of the chemical structure of Chloryl fluoride are given below:. The 2D chemical structure image of Chloryl fluoride is also called skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of Chloryl fluoride are implied to be located at the corner s and hydrogen atoms attached to carbon atoms are not indicated — each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds. The 3D chemical structure image of Chloryl fluoride is based on the ball-and-stick model which displays both the three-dimensional position of the atoms and the bonds between them. The radius of the spheres is therefore smaller than the rod lengths in order to provide a clearer view of the atoms and bonds throughout the chemical structure model of Chloryl fluoride. For a better understanding of the chemical structure, an interactive 3D visualization of Chloryl fluoride is provided here. The Chloryl fluoride molecule shown in the visualization screen can be rotated interactively by keep clicking and moving the mouse button. Mouse wheel zoom is available as well — the size of the Chloryl fluoride molecule can be increased or decreased by scrolling the mouse wheel. The information of the atoms, bonds, connectivity and coordinates included in the chemical structure of Chloryl fluoride can easily be identified by this visualization. By right-clicking the visualization screen, various other options are available including the visualization of van der Waals surface and exporting to an image file. Our Deep Data encompasses property data, spectral data, quantum chemical data, and molecular descriptor data for a wide range of chemical compounds. It features more than 2, high-quality datasets per single chemical compound, totaling over 8 billion datasets for 4.
Transcribed Image Text: [References] a. Problem AE: Which member of the following pairs would you expect to be more energetically stable?
You can find the procedure here. So, "Cl" is the central atom. You have 20 valence electrons in your trial structure. How can I draw the Lewis structure for ClO2-? Ernest Z. Jul 15,
We draw Lewis Structures to predict: -the shape of a molecule. For the ClO2- Lewis structure the total number of valence electrons found on the periodic table for the ClO2- molecule. Once we know how many valence electrons there are in ClO2- we can distribute them around the central atom with the goal of filling the outer shells of each atom. In the Lewis structure for ClO2- we put Chlorine Cl at the center of the structure since it is the least electronegative. There are total of 20 valence electrons for the ClO2- Lewis structure. Remember that the negative sign counts as one valence electron. To show that the ClO2- Lewis structure is an ion with a -1 change we need to put brackets around the structure and put a negative side on the outside of the brackets. It is helpful if you: Try to draw the ClO 2 - Lewis structure before watching the video. Watch the video and see if you missed any steps or information. Try structures similar to ClO 2 - for more practice.
Lewis structure for clo2f
Chloryl fluoride is the chemical compound with the formula ClO 2 F. It is commonly encountered as side-product in reactions of chlorine fluorides with oxygen sources. The differing structures reflects the greater tendency of chlorine to exist in positive oxidation states with oxygen and fluorine ligands. The related bromine compound bromyl fluoride BrO 2 F adopts the same structure as ClO 2 F, whereas iodyl fluoride IO 2 F forms a polymeric substance under standard conditions. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. Download as PDF Printable version. In other projects.
Skyrim boethiahs proving
Where does hydrogen fit into the electronegativity trend for You can reuse this answer Creative Commons License. TlF TlF 3. Q: In developing the concept of electronegativity, Pauling usedthe term excess bond energy for the…. A: Given: Electronegativity and Bond Polarity. Problem 90E Problem 91E: Benzene C6H6 consists of a six-membered ring of carbon atoms with one hydrogen bonded to each There are certain rules…. A: The species which have same no. A: For drawing resonating structures, just make the shifting of electrons i. Problem 39E: State whether or not each of the following has a permanent dipole moment.
ClO2 is the molecular formula of Chlorine dioxide that is commonly used to treat potable water.
WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. The bond lengths of the N-N bonds in the… A:. PbF 2 PbF 4. How many different types of resonance structures can be drawn for the…. The molecular formula of Chloryl fluoride is available in chemical formula page of Chloryl fluoride , which identifies each constituent element by its chemical symbol and indicates the proportionate number of atoms of each element. Oxygen O has an atomic mass of about Include an estimate TeF 4 TeF 6. Include resonance structures. Justify each The 2D chemical structure image of Chloryl fluoride is also called skeletal formula, which is the standard notation for organic molecules. Confirm Valid email address confirmed. There are 3 non-H bond s , 2 multiple bond s , and 2 double bond s.

I apologise, but, in my opinion, you are not right. I am assured. Let's discuss it.
I recommend to you to come for a site on which there are many articles on this question.