Lewis dot structure for hcn
We'll put the Carbon in the center, because it's amosdoll music electronegative than the Nitrogen, and Hydrogens always go on the outside of Lewis structures. We have a total of ten valence electrons for the HCN Lewis structure. We'll put two between atoms to lewis dot structure for hcn chemical bonds, so we've used four, then we'll go around the Nitrogen, six, eight, and ten. So when we look at the Lewis structure, Nitrogen had eight valence electrons, but the Carbon only has four.
Draw the Lewis structure of HCN. Draw the Lewis structure of B e C l 2. Write the Lewis dot structure of C O molecule. Draw the Lewis dot structure of Hydrogen sulphide molecule. Draw the Lewis structure of the species as mentioned below: An odd electron molecule is formed. Draw the Lewis structure of C l O 4 per chlorate ion.
Lewis dot structure for hcn
There is a formal negative charge associated with this anion. Where does it reside? The nitrogen nucleus has 3 electrons from the triple bond, and 2 electrons from its lone pair, and 2 inner core electrons; the associated charge balances the 7 protons in the nitrogen nucleus, so the nitrogen is formally neutral. The carbon atom has or shares 3 electrons from the triple bond, and a lone pair of electrons, which it owns. With 2 inner core electrons, this makes 7 electrons with which it is associated. Since, the atomic number of carbon is 6 , the carbon atom is formally negatively charged. What is the Lewis structure of HCN? Oct 29, Explanation: The nitrogen nucleus has 3 electrons from the triple bond, and 2 electrons from its lone pair, and 2 inner core electrons; the associated charge balances the 7 protons in the nitrogen nucleus, so the nitrogen is formally neutral. Related questions How is the Lewis structure of an ion written?
There is a formal negative charge associated with this anion. What are the units used for the ideal gas law? Since the central tin atom does not complete octet, a double bond is formed between each terminal oxygen atom.
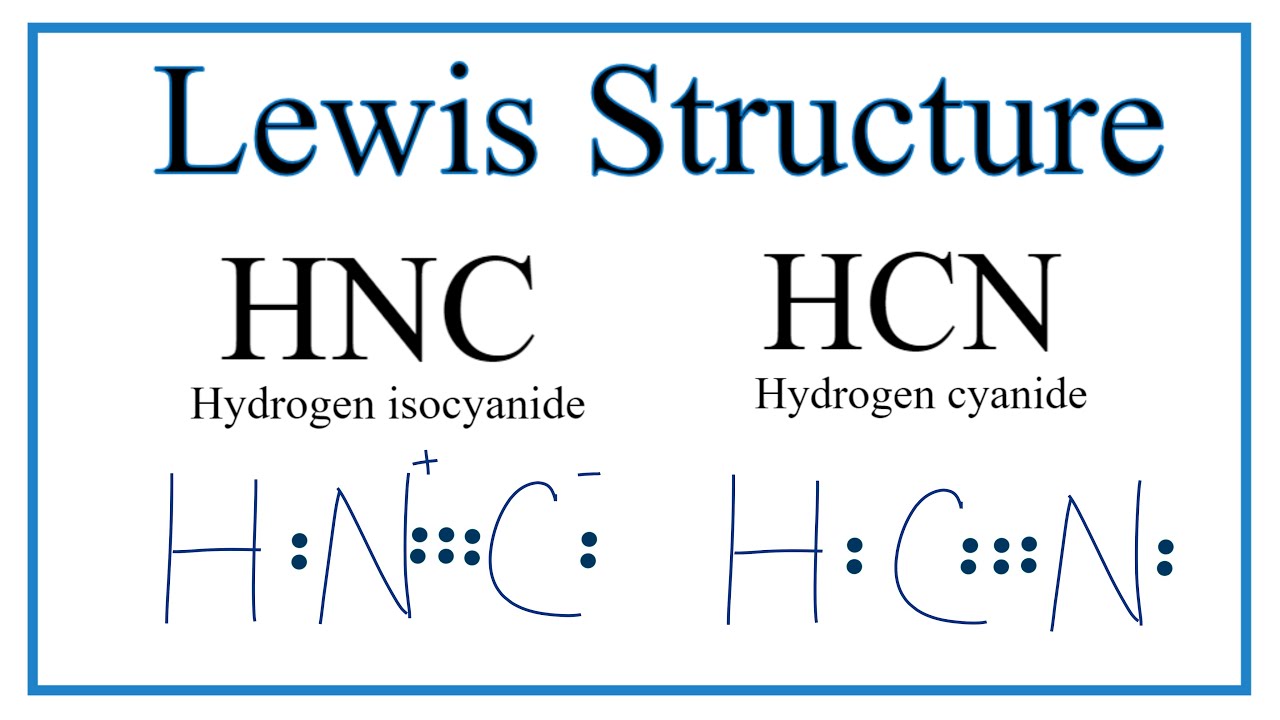
H-CN: Hydrogen forms a single bond with Carbon and carbon a triple bond with nitrogen, with 1 lone pair on the other side of N. There is a single covalent bond between the hydrogen and carbon atom, represented by two dots, : , each of which represents a shared electron; a triple covalent bond between the carbon and nitrogen atom, represented by three pairs of dots, , representing three pairs of shared electrons, and a lone pair of electrons on the nitrogen atom, represented by a pair of dots, :. I need help with lewis diagram of HCN - which one do I put in the middle? Jared Vincent L. Feb 23, The least electronegative atom is usually the one in the middle, in this case C.
Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Represented by the chemical formula, HCN is one of those molecules that has an interesting Lewis structure. This liquid is used in electroplating, mining, and as a precursor for several compounds. Keep reading this post to find out its shape, polarity, and more. First, let us look at its Lewis dot structure and the valence electrons that participate in forming bonds. To draw the Lewis dot structure of any molecule, it is essential to know the total number of valence electrons in the structure. To know the valence electrons of HCN, let us go through the valence electrons of individual atoms in Hydrogen Cyanide.
Lewis dot structure for hcn
Hydrogen Cyanide is a very toxic acid and is famous for causing irritation in the eyes and respiratory system if any human inhales HCN in substantial quantity. HCN has a very strong and pungent smell which is not favorable for humans. The smell can be categorized as being that of bitter almonds. It is considered to be a dangerous and poisonous substance that is stored carefully to avoid any leaks or combustion because the storage containers if exposed to extreme heat might cause explosions.
Merve pastanesi ankara
Publisher: Cengage Learning. See the Big List of Lewis Structures. So it is taken as the central atom with 3 fluorine atoms at the terminal positions of it The nitrogen has 5 and each fluorine atom have 7 valence electrons. Out of butyne or butene which has higher dipole moment? Valence bond theory VBT in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. Show all chapter solutions add. See similar textbooks. Valence Bond Theory Vbt. Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts! Boron atom has less electronegativity comparing to fluorine. Example 9. Goode, David W. Impact of this question views around the world. The geometry of the ion is that of an equilateral Polarity Of Water.
.
Since there are 3 fluorine atoms the total valence electron of the molecule becomes Draw the structure and state polar and non-polar nature of a Ch Write a Lewis structure for Which of these molecules have octet and which are exceptions, include the type of exception, if having any: NO2, SCl2, NH3? Answer to Problem 9. The nitrogen nucleus has 3 electrons from the triple bond, and 2 electrons from its lone pair, and 2 inner core electrons; the associated charge balances the 7 protons in the nitrogen nucleus, so the nitrogen is formally neutral. So let's move another pair to the center. To obtain the remaining electrons 12, two electrons for each bond is reduced from the total number of valence electrons, which then further distributed on the terminal oxygen atoms to fill the octets. What is the electron dot diagram for carbon? Interpretation Introduction. Is the octet rule Want to see more full solutions like this? Draw the Lewis structure of C l O 4 per chlorate ion. It gives a quantum mechanical approach to the forma….

Bravo, excellent phrase and is duly
I can not participate now in discussion - it is very occupied. I will return - I will necessarily express the opinion.
Full bad taste