Lewis dot of h2s
Hydrogen sulfide H2S consists of two hydrogen H atoms and one sulfur S atom. Sulfur is located in group 16 of the periodic table, indicating that it has six valence electrons, while hydrogen belongs to group 1 and brings one valence electron per atom.
Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. In all cases, the same types of diagrams are used to indicate where electrons and bonds are located. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules. An octet rule governs Lewis structures. Lewis structures are useful for understanding chemical bonding.
Lewis dot of h2s
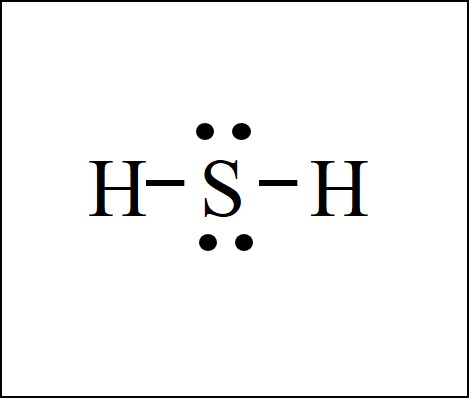
There are 2 single bonds between the Sulfur atom S and each Hydrogen atom H. There are 2 lone pairs on the Sulfur atom S. In order to find the total valence electrons in H2S molecule , first of all you should know the valence electrons present in hydrogen atom as well as sulfur atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Sulfur is a group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now here the given molecule is H2S dihydrogen sulfide and it contains hydrogen atoms H and sulfur atom S.
The Lewis structure of H2S represents the arrangement of atoms and valence electrons in a hydrogen sulfide molecule. Distributing Remaining Valence Electrons. You can see the electronegativity values of hydrogen atom H and sulfur atom S in the above periodic table.
The Lewis structure of H2S consists of a central sulphur atom S and two external hydrogen atoms H at a The sulphur atom S and the two hydrogen atoms H are each connected by a single bond. The Lewis structure of H2S is shown below:. Sulphur and hydrogen are elements of group 16 and group 1 of the periodic table, respectively. Therefore, there are 6 valence electrons in a sulphur atom and 1 valence electron in a hydrogen atom.
H2S or hydrogen sulfide gas is colorless in nature. With many other various pet names like sour gas, sewer gas, etc this gas is poisonous and corrosive as well. I am sure you are not expecting a good odor from this gas! Well yes, you are right, hydrogen sulfide gas smells like rotten eggs!! The molar mass of H2S is H2S has a covalent bond because the sulfur atom completes its octet by sharing 2 electrons with 2 hydrogen atoms and thus forms a covalent bond. I have also written specifically on it, check out the post on the covalent bonds of H2S. First and foremost it is important to determine how many valence electrons are present in the compound. The central atom is basically the atom with the highest number of bonding sites. Here, the central atom will be the sulfur atom.
Lewis dot of h2s
Transcript: All right, this is Dr. On the periodic table: Hydrogen, group 1, has 1 valence electron, but we have two Hydrogens here so let's multiply that by 2. Plus Sulfur is in group 6 or 16 on the periodic table, so it has 6 valence electrons. Total of 8 valence electrons. Let's draw this thing. We'll put Sulfur here. Hydrogen always goes on the outside, we'll put it out there. Now we want to take some of these valence electrons and spread them around the atoms.
Shemale and shemale
For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside. An octet rule governs Lewis structures. Hydrogen Sulfide-Hazard and Toxicity Sep 9, Hydrogen Sulfide is a colorless, very poisonous, flammable gas with the characteristic foul odor of rotten eggs. This indicates that the above lewis structure of H2S is stable and there is no further change in the above structure of H2S. Formal charges aid in determining the most plausible Lewis structure. Step 4 Stability of the structure In order for the central sulphur S atom to be stable, we must check that it has an octet. Identify the Central Atom. Learn more. These outer hydrogen atoms are forming a duplet and hence they are stable.
Also, there are two lone pairs around sulfur atom.
Learn more. Sulphur and hydrogen are elements of group 16 and group 1 of the periodic table, respectively. The Lewis structure of H2S consists of a central sulphur atom S and two external hydrogen atoms H at a Valence electrons are the electrons that are present in the outermost orbit of any atom. This results in a stable and balanced initial structure. For the H2S molecule, the total number of electron pairs is 4. These outer hydrogen atoms are forming a duplet and hence they are stable. You can see the electronegativity values of hydrogen atom H and sulfur atom S in the above periodic table. Distributing Remaining Valence Electrons. H2S does not exhibit resonance structures since there are no multiple bond placements or delocalized electrons within the molecule. Besides regulating the acidity of food, it is also used as an antimicrobial food preservative and a Daphnia magna metabolite.

I confirm. I join told all above. We can communicate on this theme. Here or in PM.
Well, well, it is not necessary so to speak.
I can not participate now in discussion - it is very occupied. But I will return - I will necessarily write that I think on this question.