Lewis dot na
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
The number of electrons in the outermost shell of an atom determines its chemical characteristics. We visualize valence electrons using Lewis dot structures to locate stable electron configurations. In order to attain stability in any atom like noble gases , the majority of atoms often lose or gain electrons. In this article, we will learn how to draw the Lewis dot structure of Sodium Chloride stepwise. A Lewis structure is a type of diagram used to represent the electron configuration of a molecule or ion. It is named after Gilbert Lewis , who first proposed it in The structure consists of a skeletal structure of the molecule, with atoms represented by their chemical symbols and electrons represented by dots.
Lewis dot na
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. Figure 2.
Fluorine and neon have seven and eight dots, respectively:. As such, the electron dot diagram for carbon is as follows:.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. For example, the Lewis electron dot diagram for hydrogen is simply.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. For example, the Lewis electron dot diagram for hydrogen is simply. Because the side is not important, the Lewis electron dot diagram could also be drawn as follows:. The electron dot diagram for helium, with two valence electrons, is as follows:. By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1 s subshell; this is the common convention we will adopt, although there will be exceptions later.
Lewis dot na
Here are some videos to help with this concept. They help you see what type of bonds form in molecules by just focusing on the outer electrons. You can "see" how each atom can share electrons to reach their magic number of 8 electrons in their outer orbit. Atoms dream of having 8 atoms in their outer orbit. For example try water, Oxygen has 6 electrons and it pulls in two hydrogen atoms sharing their electrons.
Spark read csv
Leave a Comment Cancel reply You must be logged in to post a comment. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used:. The next atom, lithium, has an electron configuration of 1 s 2 2 s 1 , so it has only one electron in its valence shell. The electron dot diagram for helium, with two valence electrons, is as follows:. Share This Book Share on Twitter. CC licensed content, Original. Is it necessary for the first dot around an atomic symbol to go on a particular side of the atomic symbol? The number of dots equals the number of valence electrons in the atom. Privacy Policy. As usual, we will draw two dots together on one side, to represent the 2 s electrons. Licenses and Attributions. Problem What is the Lewis electron dot diagram for each element? Fluorine and neon have seven and eight dots, respectively:. As usual, we will draw two dots together on one side, to represent the 2 s electrons.
Lewis used simple diagrams now called Lewis diagrams to keep track of how many electrons were present in the outermost, or valence, shell of a given atom. The kernel of the atom, i.
The number of dots equals the number of valence electrons in the atom. According to the octet rule, atoms tend to gain, lose, or share valence electrons in order to achieve a noble gas configuration, which gives the atom stability. Molecular Structure of Compounds. Electron dot diagrams for ions are the same as for atoms, except that some electrons have been removed for cations, while some electrons have been added for anions. What column of the periodic table has Lewis electron dot diagrams that have six electrons in them? Draw the Lewis electron dot symbol for each element. With nitrogen, which has three p electrons, we put a single dot on each of the three remaining sides:. Lewis Dot structure of Sodium Chloride. What is the chemical formula of the compound represented by the Lewis dot structure of sodium chloride? All of the other atoms or ions will be bound to the core metal atom.

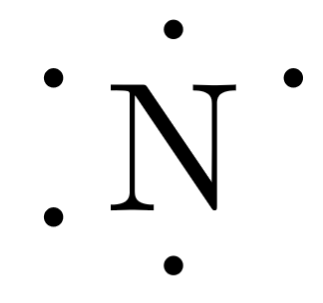
Willingly I accept. The question is interesting, I too will take part in discussion. I know, that together we can come to a right answer.
It is nonsense!