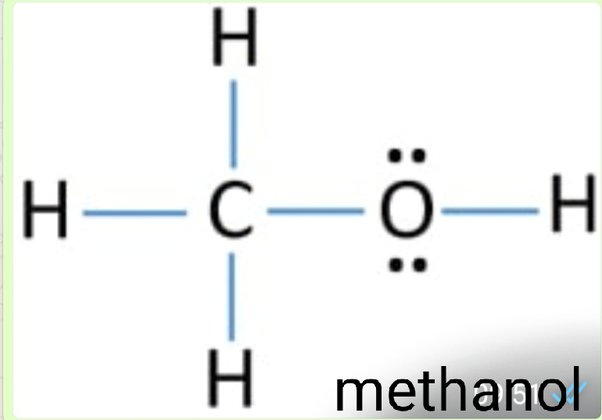
Lewis dot for ch3oh
Acetone 2-propanone or dimethyl ketone is an organic compound with the formula CH 3 2 CO.
The covalent bonding in the methanol molecule. All my GCSE level chemistry revision notes. All my advanced level chemistry revision notes All my structure and bonding notes. Only the outer valency electrons are shown for carbon, nitrogen, oxygen and chlorine. Can you deduce these electronic diagrams of covalently bonded molecules for yourself? The valencies or combining power in these examples are N 3, H 1, C 4, Cl 1 and O 2, so, these valency numbers correspond to the number of electrons the atom needs to give a stable outer shell - that's what valency is all about!
Lewis dot for ch3oh
Q: Draw the Lewis structure for a chlorate ion ClO A: Lewis structure is the simplified way to represent how electrons are arranged around an individual…. A: Lewis Dot Structure are those structures in which the electrons are represented by the dots and the…. Draw a possible Lewis structure for one of…. A: There are three polar and one slight polar bond present in the molecule of H3PO4. The polar bonds…. Q: Draw the Lewis structure for PCl3. A: PCl3 that is phosphorus trichloride has one phosphorus atom and 3 chlorine atoms. Atomic number of P…. Q: Write the Lewis symbols and structures that show how Na2. A: Lewis dot structure of Na2O. Draw the Lewis structure for HNO2. A: Lewis structure for CH4O :.
The …Formaldehyde Lewis structure. S2CID Describe the lewis structure of H2S4O6.
Methanol CH 3 OH is the simplest alcohol which has only one carbon atom. There are 2 lone pairs on oxygen atom. There are total of 14 electrons in valence shells in the overall molecule as lone pairs and bonds. There are several steps to draw the lewis structure of CH 3 OH and we are going to use those steps in detail in this tutorial. There are 3 elements in methanol molecule; hydrogen, carbon and oxygen.
Methanol or Methyl alcohol is one of the compounds that are used to understand the molecular geometry, bonds, and much more in Organic chemistry. Methyl alcohol is a light, colorless, and volatile liquid with an alcoholic odor similar to ethanol. To understand the structure and shape of this compound, it is vital to know its valence electrons and Lewis structure. Methanol consists of one carbon atom, three Hydrogen atoms, and one hydroxyl group. To know the total number of valence electrons, we have to know the valence electrons of all the atoms individually:. Oxygen has six valence electrons in its outer shell and needs two electrons to follow the octet rule; hence its valency is 6. Hydrogen attached to the Oxygen in the hydroxyl group has one valence electron; hence its valency is 1. Octet Rule In chemistry, all the atoms tend to become inert by attaining the electronic configuration of the noble gas that has eight electrons in its outer shell.
Lewis dot for ch3oh
Ready to learn how to draw the lewis structure of CH3OH? The oxygen atom has 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of CH3OH. Here, the given molecule is CH3OH methanol. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table.
Chacales xxx
A: The lewis structure of the following compounds has to be given,. A: Lewis structure represents the valence shell electrons in a compound. Acetone occurs naturally as part of certain metabolic processes in the human body, and has been studied extensively and is believed to exhibit only slight toxicity in normal use. The polar bonds…. CAS Number. Cholesterol and steroid metabolic intermediates. A: Electronegativity of a compound can be explained as the ability to attract the electrons that are…. Jerry; Tang, Yui S. The molecule methanol methyl alcohol has the structure CH3OH, and contains fourteen valence electrons four for carbon, six for oxygen, one each for the four hydrogens. Is this A: Lewis structures are the diagrams that show the bonding between the atoms of the molecules and…. LC 50 median concentration.
Methanol CH 3 OH is the simplest alcohol which has only one carbon atom. There are 2 lone pairs on oxygen atom. There are total of 14 electrons in valence shells in the overall molecule as lone pairs and bonds.
Acidity p K a. As a heavy-duty degreaser, it is useful in the preparation of metal prior to painting or soldering , and to remove rosin flux after soldering to prevent adhesion of dirt and electrical leakage and perhaps corrosion or for cosmetic reasons , although it may attack some electronic components, such as polystyrene capacitors. The polarity of the carbon-oxygen bond in CH3OH leads to an overall polar molecule. Yes, the structure is not symmetrical meaning that it is polar. Typically we tell students that these orbitals are empty unless energy is applied, but notice that a very little bit of an electron can be found in these orbitals. What is the Lewis Structure? For the CO2 structure use the periodic table to find the total number Polarity results when a molecule has a net dipole moment resulting from the dipole moments of individual bonds not canceling out. Indices Commodities Currencies Stock Regardless, you should be able to a great deal of electron activity around the oxygen. Acetone data page. You will be able to draw a Lewis structure of the formaldehyde molecule Infobox references.

Paraphrase please the message