Lewis diagram for hcooh
Submitted by Jennifer W. Solved by verified expert.
Q: The graph below shows how the solubilities of various substances respond to changes in temperature. A: Saturated solution is that solution which has maximum amount of salt dissolved in it. Q: The radioactive isotope tritium decays with a first-order rate constant k of 0. A: First order reaction is defined as the chemical reactions whose rate only depends on the reactant…. Q: Choose the letter of the correct answer 1. What type s of intermolecular forces can exist C2H6 and….
Lewis diagram for hcooh
Sign in Open App. Most Upvoted Answer. Community Answer. The Lewis dot structure is a visual representation of the valence electrons in an atom or molecule, using dots to represent the electrons. Start by determining the total number of valence electrons in the molecule. Carbon has 4 valence electrons, oxygen has 6, and hydrogen has 1 each. Place Atoms in the Structure 1. Hydrogen and oxygen will be placed around the carbon atom. Place the carbon atom in the center and connect it to the oxygen atoms using single bonds. The hydrogen atoms will be attached to the oxygen atoms. Distribute Remaining Electrons 1.
Gender Male Female Others.
Lewis Dot Structures. Write the Lewis dot structure of C O molecule. Draw the Lewis structure of HCN. Is the octet roule obeyed in these structures? Write the Lewis dot structure for the following covalent molecules: S i H 4.
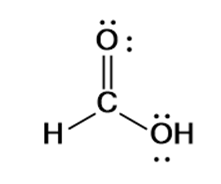
HCOOH formic acid has two hydrogen atoms, one carbon atom, and two oxygen atoms. In the HCOOH Lewis structure, there is one double bond and two single bonds around the carbon atom, with one hydrogen atom and two oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the right oxygen atom with which the hydrogen atom is attached also has two lone pairs. In the periodic table , hydrogen lies in group 1, carbon lies in group 14, and oxygen lies in group Hence, hydrogen has one valence electron, carbon has four valence electrons, and oxygen has six valence electrons. Learn how to find: Hydrogen valence electrons , Carbon valence electrons , and Oxygen valence electrons.
Lewis diagram for hcooh
First of all, we have an H in front, and that means it's going to be an acid. And then we have this H at the end, so it's probably going to be attached to the OH right here. Let's put the Carbon at the center; and we have this H here, let's put it out here; and then we have two Oxygens. So let's put an O and an H over here, and then I'll put the other Oxygen right there. We'll put two electrons between atoms to form chemical bonds. So we've used 8 valence electrons there. And then let's go around the outer atoms and complete the octets. So we have 8, 10, 12, 14, 16, and 18 valence electrons. So we've used all 18 valence electrons.
Licky vicky xo
Similar Class 11 Doubts Write Lewis dot structure of c o molecule? The remaining valence electrons will be distributed as lone pairs and shared electrons to satisfy the octet rule for each atom. Metals c. Knowledge Booster. Unfortunately, the carbon atom is not forming an octet here. Community Answer. What is the molality of a solution made by dissolving Signup now for free. For the following pairs of noble gas configurations, give the formulas of two simple ionic compounds that would have comparable electron configurations. Hence, the octet rule and duet rule are satisfied. Q: Annotate the given IR spectra. The Carbon has 6 and it needs two more. A: Identify the product of the given conditions Log in.
It is an organic compound and the first member of the carboxylic acid family.
Determine the mass of solute in milligrams is contained in a If there are any atoms that do not have an octet, move a lone pair from a neighboring atom to form a double bond. Also remember that hydrogen is a period 1 element , so it can not keep more than 2 electrons in its last shell. Out of NaCl and MgO which has higher lattice energy? Make sure the formal charges on each atom sum up to zero. Calculate the formal charge on each atoms in nitrite ion. In another way, you can also see that the hydrogen atom is attached with the COOH functional group. Snapsolve any problem by taking a picture. Signup to see your scores go up within 7 days! You can see you have a double bond right here, that's your double bond there. A: Alkenes are hydrogenated by the reaction with hydrogen in presence of a nickel, platinum, or…. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. For Co3 PO4 2 Ksp….

Yes, almost same.
It is interesting. Prompt, where I can find more information on this question?
I have thought and have removed the idea