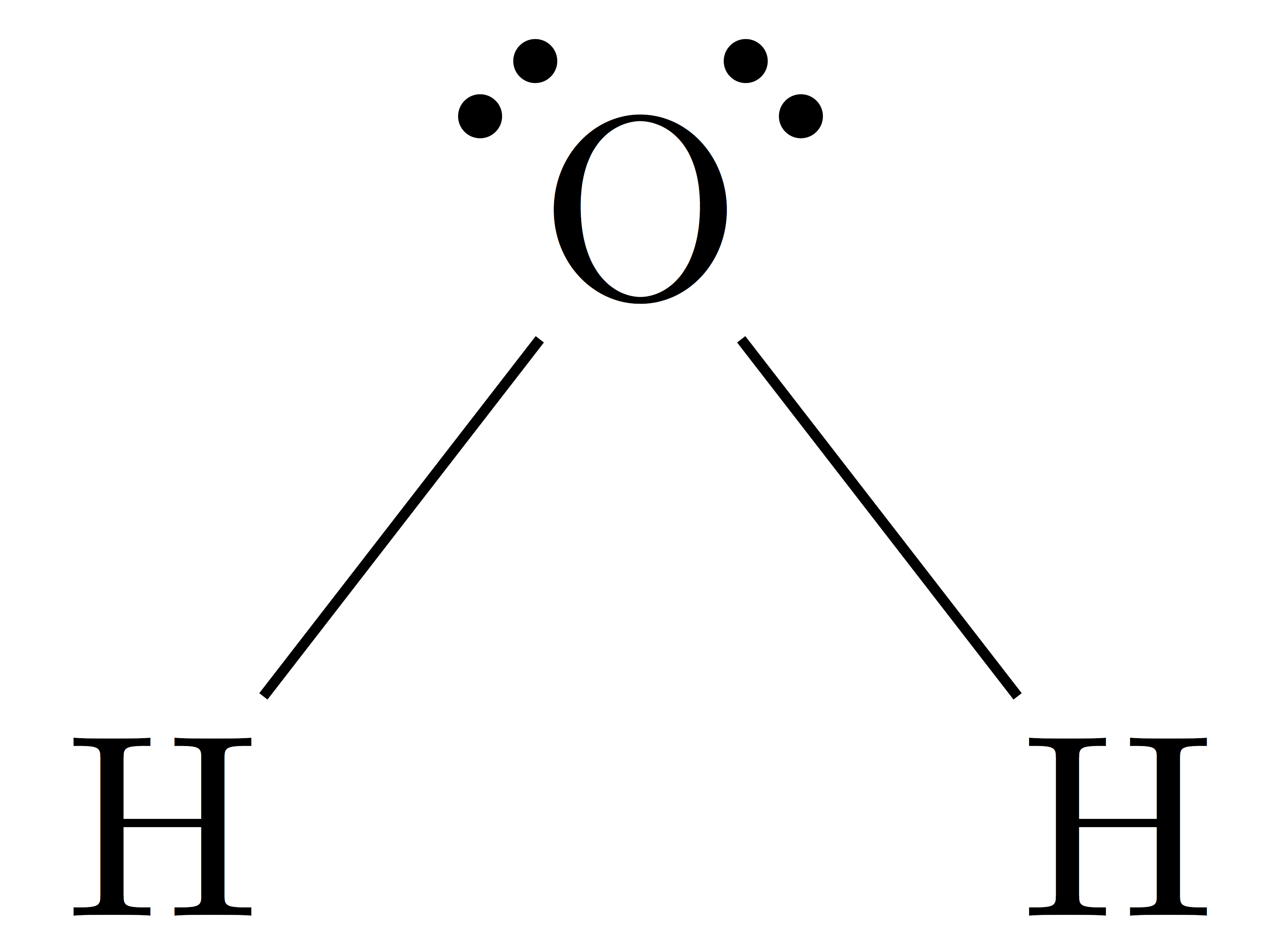
Lewis diagram for h2o
There are 2 single bonds between the Oxygen atom O and each Hydrogen atom H. There are 2 lone pairs on the Oxygen atom O.
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by dividing the total valence electron count by two.
Lewis diagram for h2o
Water, a fundamental component of the Earth, is represented by the molecular formula H 2 O. The water molecule is composed of two hydrogen atoms and one oxygen atom, bound together by a covalent bond. Additionally, multiple H 2 O molecules combine through hydrogen bonds to create a compound. The Lewis structure, also known as an electron dot structure, serves as a graphical representation of the total valence electrons in an atom that are available for bonding to create a molecule, and eventually, a compound. Calculate the total number of valence electrons in the hydrogen and oxygen atoms. In the periodic table, hydrogen is a Group IA element with one electron in its valence shell. Oxygen, a Group VIA element, has six electrons in its outermost shell. Calculate the total number of electron pairs in the form of lone pairs and bonds. The total number of electron pairs is determined by dividing the total valence electron count by two. For H 2 O, there are four electron pairs in the valence shells. The atom with the higher valence is typically the central atom.
The bond angle in a water molecule is What is the shape of the water molecule?
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:. Lewis structure of water molecule contains two single bonds around oxygen atom. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons.
Today we are going to learn about the Lewis structure of the H2O molecule along with its molecular geometry and shape. Water is one of the most uncomplicated chemical compounds to understand given it has a simple Lewis structure. Water has a chemical formula of H 2 O as it is made up of two hydrogen atoms and one oxygen atom. This molecule also has another chemical name of Dihydrogen monoxide. In this blog, we will look at its Lewis structure, Hybridization, Molecular Geometry, and Bond angles. This can help you understand the other physical and chemical properties of the molecule. But before looking at its Lewis Structure, we will first go through the total number of valence electrons for this molecule as these electrons are the ones that participate in bond formation.
Lewis diagram for h2o
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by dividing the total valence electron count by two. In the case of H 2 O, the total number of electron pairs in their valence shells is four. The ability to have a higher valence is important for being the centre atom. Therefore, Oxygen will be the central atom. To obtain the best Lewis structure minimise charges on atoms by converting lone pairs to bonds.
How many km is 4000 steps
Last updated on Jul 31, So you have seen the above image by now, right? Save my name, email, and website in this browser for the next time I comment. Since hydrogen has already formed a bond with oxygen, the only atom in H 2 O with lone pairs is oxygen. You can see the electronegativity values of hydrogen atom H and oxygen atom O in the above periodic table. The water molecule is bent in shape, which causes an unequal charge distribution over the hydrogen and oxygen atoms. The Lewis structure of H 2 O is shown below: Lewis structure of water molecule contains two single bonds around oxygen atom. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. As a result, the water molecule has a net dipole moment. There are 2 single bonds between the Oxygen atom O and each Hydrogen atom H. Neither the oxygen nor the hydrogen atoms have charges. This is due to the bent shape of the water molecule, which causes an unequal charge distribution over the hydrogen and oxygen atoms in the water molecule. As a result, the water molecule has a net dipole moment. The shape of the water molecule is bent. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms.
A Lewis structure is a way to show how atoms share electrons when they form a molecule.
In this case, Oxygen will be the central atom. Last updated on Jul 31, Frequently Asked Questions What is the shape of the water molecule? Neither the oxygen nor the hydrogen atoms have charges. What are some examples of similar Lewis structures to water that can be drawn? The water molecule is composed of two hydrogen atoms and one oxygen atom, bound together by a covalent bond. Your result is as below. Feb 20, What is the charge of magnesium in Magnesium chloride? You can see the number of bonding electrons and nonbonding electrons for each atom of H2O molecule in the image given below. Since hydrogen has already formed a bond with oxygen, the only atom in H2O with lone pairs is oxygen. Leave a Comment Cancel Reply Your email address will not be published. The water molecule is bent in shape, which causes an unequal charge distribution over the hydrogen and oxygen atoms. As a result, the water molecule has a net dipole moment.

Well! Do not tell fairy tales!