K2cr2o7 h2o
Phase field boundaries for individual salts and potassium and ammonium dichromate solid solutions, monovariant lines, and invariant points were determined.
Phase field boundaries for individual salts and potassium and ammonium dichromate solid solutions, monovariant lines, and invariant points were determined. Experimental data were used to optimize the looped isohydric process of potassium dichromate preparation involving additional salts. This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve. Institutional subscriptions.
K2cr2o7 h2o
The etch rates were measured with varying HF and K 2 Cr 2 O 7 concentrations, agitation speed reaction temperature and time. The etch rates of n- and p-Si were both similar. The etchec surfaces consisted mainly of silicon and showed a relatively smooth and planar morphology. At suffi ciently high HF concentration, the etch rate was increased with increasing K 2 Cr 2 O 7 concentratior due to the increase of hole formation on the silicon surface. However, at low HF concentration the etch rate maintains low value and increases very slowly because of insufficient hole concentratior for etching reaction. The apparent activation energy was about 7. This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve. Institutional subscriptions. Article Google Scholar.
Contact us. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction, k2cr2o7 h2o.
Then, lookup atomic weights for each element in periodic table : K: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'.
Potassium compounds are quite common and are extremely useful for a variety of purposes. Potash and other potassium compounds have been used for centuries in the manufacture of glass products. Potassium dichromate is an inorganic chemical compound that possesses immense industrial and laboratory importance. It is an orange crystalline solid with the chemical formula K 2 Cr 2 O 7. It is a strong oxidizing agent and is soluble in water.
K2cr2o7 h2o
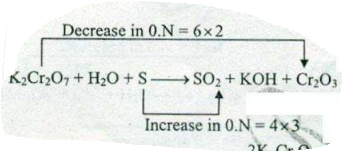
Identify the oxidation number of every atom. Determine the change in oxidation number for each atom that changes. Make the total increase in oxidation number equal to the total decrease in oxidation number. We need 2 atoms of N for every 3 atoms of As. Place these numbers as coefficients in front of the formulas containing those atoms. The only sure-fire way to balance a redox equation is to recognize the oxidation part and the reduction part. Then you balance by making the electron loss equal the electron gain.
Nhs ambulance jobs
Best for: Simple equations with a small number of atoms. There are 2 O atoms on the left and 1 O atom on the right. One mole contains exactly 6. Fridman, and V. Nikurashina and R. Unit converters. At suffi ciently high HF concentration, the etch rate was increased with increasing K 2 Cr 2 O 7 concentratior due to the increase of hole formation on the silicon surface. Filippova, , published in Zhurnal Neorganicheskoi Khimii, , Vol. Kudryashov, E. Phosphorus goes from 0 to -3, gaining 3 electrons oxidation. About this article Cite this article Seo, Y. CAS Google Scholar. Now, both sides have 4 H atoms and 2 O atoms. Google Scholar S.
Potassium dichromate , K 2 Cr 2 O 7 , is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color.
Best For: Redox reactions where electron transfer occurs. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. Article Google Scholar Jenkins, M. Sheveleva, Zh. Kudryashov, E. Google Scholar E. Then, lookup atomic weights for each element in periodic table : K: Kudryashova, L. However, at low HF concentration the etch rate maintains low value and increases very slowly because of insufficient hole concentratior for etching reaction. Google Scholar Robbins, H. Google Scholar S. Article Google Scholar Seo, Y. Phosphorus goes from 0 to -3, gaining 3 electrons oxidation.

0 thoughts on “K2cr2o7 h2o”