J kgk to j kgc
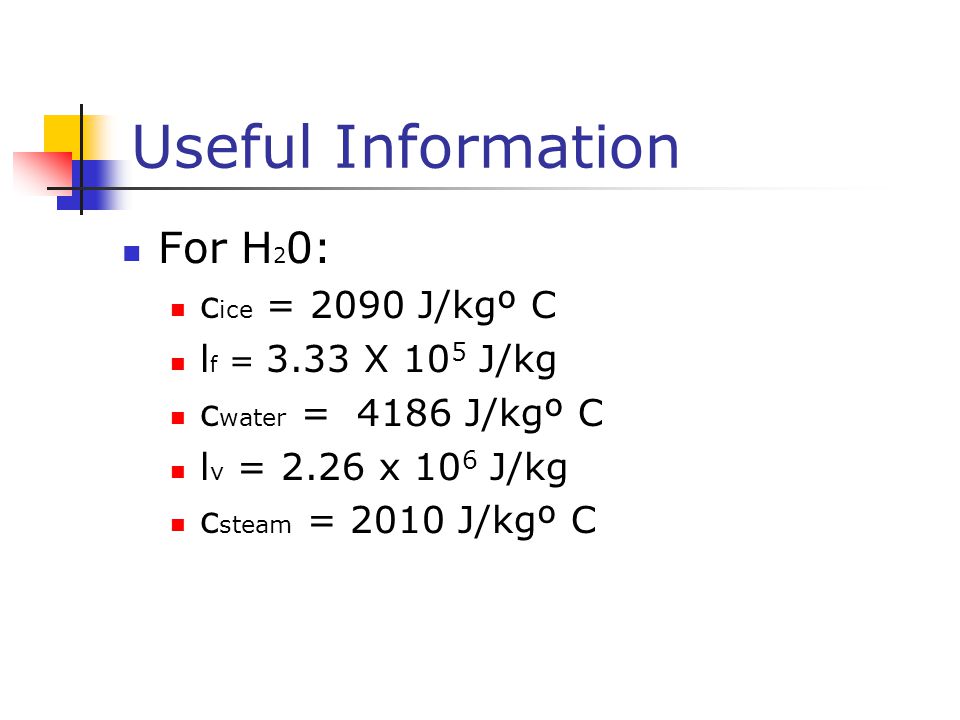
Specific Heat is the j kgk to j kgc of heat required to change one mass unit of a substance by one degree in temperature. Value use period as decimal point. Use the links to see tabulated values of specific heat of gasescommon liquids and fluidsfood and foodstuffmetals and semimetalscommon solids and other common substances.
Random converter. Click or tap to find out! Heat causes molecules to move, and this movement is called molecular diffusion. The greater the temperature in a substance — the more the molecules move, and the higher the rate of diffusion. The movement of molecules also depends on a range of other factors, such as pressure, the viscosity of the substance, its concentration, resistance to diffusion, the distance that a molecule travels in order for the diffusion to occur, and the mass of a molecule.
J kgk to j kgc
Value to be converted:. Next enter the value you want to convert. Then, when the result appears, there is still the possibility of rounding it to a specific number of decimal places, whenever it makes sense to do so. In so doing, either the full name of the unit or its abbreviation can be used. Then, the calculator determines the category of the measurement unit of measure that is to be converted, in this case 'Specific heat capacity'. After that, it converts the entered value into all of the appropriate units known to it. In the resulting list, you will be sure also to find the conversion you originally sought. For this alternative, the calculator also figures out immediately into which unit the original value is specifically to be converted. Regardless which of these possibilities one uses, it saves one the cumbersome search for the appropriate listing in long selection lists with myriad categories and countless supported units. All of that is taken over for us by the calculator and it gets the job done in a fraction of a second. Furthermore, the calculator makes it possible to use mathematical expressions. But different units of measurement can also be coupled with one another directly in the conversion.
The latter is because water evaporation requires a large amount of energy. Inedible insulators are also sometimes used in cooking.
.
Convert heat capacity units, including joule per Kelvin, calorie per Celsius and BTU per Fahrenheit, with our heat capacity measurement units conversion tool. Input a value in the provided field and choose the heat capacity units you wish to convert between. Specific heat capacity is the amount of heat required to raise the temperature of a particular substance of mass kilogram, gram, pound by 1 degree celsius, fahrenheit, kelvin. It means that the heat energy required to raise the water's temperature by 1 celsius is joules per kilogram. For specific heat capacity comparision of materials, please visit specific heat capacity table. For heat transfer coefficient units converter, please visit heat transfer coefficient converter. For heat flux density units converster, please visit heat flux density converter. How to use the converter? Enter Value. Number of significant figures: Ex: Result is
J kgk to j kgc
Specific Heat is the amount of heat required to change one mass unit of a substance by one degree in temperature. Value use period as decimal point. Use the links to see tabulated values of specific heat of gases , common liquids and fluids , food and foodstuff , metals and semimetals , common solids and other common substances. Download and print Specific Heat Converting Chart. Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with older versions of the amazing SketchUp Make and the newer "up to date" SketchUp Pro. Translate this page to Your Own Language. If you want to promote your products or services in the Engineering ToolBox - please use Google Adwords. Temperature o C K o F. Length m km in ft yards miles naut miles.
Penndot traffic cam
Because of this their specific heat capacity is very low. Microwave Ovens The effectiveness of heating foods in a microwave oven depends, among other things, on the specific heat capacity of the products used. We use such materials for cups and plates, especially if they are meant for hot foods. Soup stays hot for a long time in a ceramic bowl Cooking Utensils We select materials based on heat capacity if we intend to use them to make everyday items such as pots and pans, tableware, and other objects that are subject to heat during their use. This often depends on the amount of water that makes up that food, but other factors are also at play. Materials with high heat capacity require more energy than those with low heat capacity, therefore if an object with low heat capacity and an object with high heat capacity are heated with the same amount of energy under the same conditions, then the temperature of the object with lower heat capacity will increase faster. This article discusses both heat capacity and specific heat capacity because the two are related. Mass is not considered when calculating heat capacity. If we want to increase diffusion by raising the temperature of a substance, for example by heating it, we will have to generate energy to produce this heat. Specific heat capacity or specific heat is the heat required to raise the unit mass of a substance by unit temperature interval under specified conditions, such as constant pressure. On the other hand, planets that do not have such extensive water coverage as does Earth, or even places on Earth with very little water, for example, deserts, have a much larger temperature fluctuation when the amount of solar heat changes. Value to be converted:. Cooling liquid meant for cold climates uses more ethylene glycol — antifreeze is one of the formulations used in this situation. When the oven is in operation, the microwaves that it emits cause the molecules in substances such as water or fats to move more frequently.
To measure, units of measurement are needed and converting such units is an important task as well.
Not only does it prevent the water from evaporating and keeping the heat inside, but it also stops the protruding parts like chicken or turkey wings from overheating and burning as a result. Value use period as decimal point. Area m 2 km 2 in 2 ft 2 miles 2 acres. This may cause the foods to brown as a result of the Maillard reaction. Heat capacity and specific heat capacity are only calculated when the object or a substance is in a steady state for example, a solid. Recommend this converter: Twitter Facebook VK. Specific Heat is the amount of heat required to change one mass unit of a substance by one degree in temperature. Often a thermometer is also used to check the temperature to determine whether the food is cooked — this is common when cooking meats or fish. Specific heat capacity for water is significantly higher than that of many other fluids because the hydrogen atoms in water molecules have very strong bonds. If it is solid once cool and it is difficult but possible to change its shape with fingers, then it is in a hard-ball stage. Often chefs use both the thermometer readings and the cold water drop method to check if the sugar is cooked to the right consistency. If we heat up several different substances with the same amount of energy, some substances may warm up at a faster rate than others, because of the factors above that affect their diffusion rate. But different units of measurement can also be coupled with one another directly in the conversion. This gives molecules less freedom to move, and less energy heat is required to make them move vigorously and to raise the overall temperature of the material.

In my opinion you are mistaken. Let's discuss. Write to me in PM.
You have hit the mark. In it something is and it is good idea. I support you.
I join. All above told the truth. Let's discuss this question. Here or in PM.