Is sf4 a polar molecule
To determine if SF 4 i spolar or not, we need to first draw its Lewis structure and determine the geometry. We came up with the following Lewis structure in the previous postso feel free to check it:.
Non-polar compounds are soluble in non -polar solvents. What is a polar and non-polar molecule? What are polar and non-polar molecules? Molecule having non-polar as well as polar bonds but the molecule as a whole is polar. Why C O 2 is non-polar but S O 2 is polar? Distinguish between polar and non-polar molecules.
Is sf4 a polar molecule
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar! Here, the "Xe-F" bond dipoles cancel each other, so the molecule is nonpolar. Chemistry Intermolecular Bonding Polarity of Molecules. Ernest Z. Apr 22, Explanation: If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. Two of the S-F bonds are pointing away from each other, and their bond dipoles cancel. But the other two "S-F" dipoles are pointing "down". Their bond dipoles do not cancel, so the molecule is polar. This is an even number, so you have to check the shape of the molecule. Hope this helps!
SF 4 molecule:. But the other two "S-F" dipoles are pointing "down".
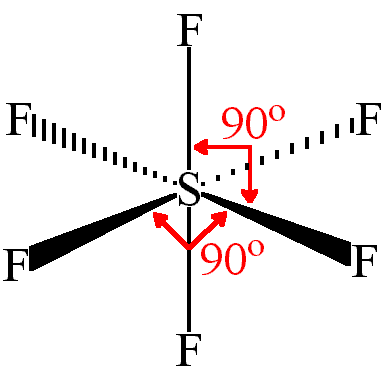
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. As a result, there are two types of F ligands in the molecule: axial and equatorial. The SF4 molecular geometry and bond angles of molecules having the chemical formula AX4E are trigonal bipyramidal. The equatorial orientations of two fluorine atoms establishing bonds with the sulphur atom are shown, while the axial locations of the other two are shown.
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space Figure 7. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance or bond length is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Valence shell electron-pair repulsion theory VSEPR theory enables us to predict the molecular structure, including approximate bond angles around a central atom, of a molecule from an examination of the number of bonds and lone electron pairs in its Lewis structure. The VSEPR model assumes that electron pairs in the valence shell of a central atom will adopt an arrangement that minimizes repulsions between these electron pairs by maximizing the distance between them. The electrons in the valence shell of a central atom form either bonding pairs of electrons, located primarily between bonded atoms, or lone pairs. The electrostatic repulsion of these electrons is reduced when the various regions of high electron density assume positions as far from each other as possible. VSEPR theory predicts the arrangement of electron pairs around each central atom and, usually, the correct arrangement of atoms in a molecule. We should understand, however, that the theory only considers electron-pair repulsions.
Is sf4 a polar molecule
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar! Here, the "Xe-F" bond dipoles cancel each other, so the molecule is nonpolar. Chemistry Intermolecular Bonding Polarity of Molecules. Ernest Z. Apr 22, Explanation: If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar.
5 letter words starting with la and ending with e
This is because the atoms will be arranged symmetrically around the central atom due to the fact that there will be no lone pairs present on the central atom. The central atom has 4 atoms connected to it, and one lone pair, therefore, the electron geometry is trigonal bipyramidal while the molecular geometry is seesaw :. Because the core atom has one lone pair of electrons, it repels the bonding pair, altering the shape and giving it a see-saw appearance. The two bonds in the axial locations will form 90 degree angles, whereas those in the equatorial positions will form degree angles. JEE Examination Scheme. If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. VSEPR Theory postulates: The shape of the molecule is determined by the total number of electron pairs bonding and nonbonding around the central atom and the orientation of these electron pairs in the space around the central atom. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. Ans : In sulphur tetrafluoride, five zones of electron density surround the core sulphur atom 4 bonds and one lone pair. Remember, the molecule is polar if it has a dipole moment, and the molecular dipole is the vector sum of all the dipoles.
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. SF4 is a chemical formula for Sulfur Tetrafluoride.
Non-polar compounds are soluble in non -polar solvents. You can also subscribe without commenting. So, there are four dipoles in SF 4 : Now, whether the molecule is polar or not will depend on the orientation of all the dipoles. Reserved Seats. Sulphur will use five orbitals: one 3s orbital, three 3p orbitals, and one 3d orbital. Post by Paul Hage 2G » Mon Nov 18, pm If the polar bonds in the following shapes are identical, with the same atoms connected to the central atom, then the molecule will be non-polar: linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral. Ans : Draw the SF Ans : In sulphur tetrafluoride, five zones of electron density surround the core sulphur atom 4 bonds and one lone pair. On each fluorine atom, there are three lone pairs. Reaction with Sulphuric Acid. JEE Application Process. CO2 will be considered as a polar molecule or non polar molecule?? The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. Frequently Asked Questions. Apr 22,

0 thoughts on “Is sf4 a polar molecule”