Is ch3oh hydrogen bonding
Central European Journal of Chemistry. The molecules of both complexes in crystal structures are linked by O—H···O hydrogen bonds, which created a three-dimensional hydrogen-bonding networks.
This definitive reference consolidates current knowledge on dihydrogen bonding, emphasizing its role in organizing interactions in different chemical reactions and molecular aggregations. After an overview, it analyzes the differences between dihydrogen bonds, classical hydrogen bonds, and covalent bonds. It describes dihydrogen bonds as intermediates in intramolecular and intermolecular proton transfer reactions. It describes dihydrogen bonding in the solid-state, the gas phase, and in solution. This is the premier reference for physical chemists, biochemists, biophysicists, and chemical engineers.
Is ch3oh hydrogen bonding
Serwis Infona wykorzystuje pliki cookies ciasteczka. Są to wartości tekstowe, zapamiętywane przez przeglądarkę na urządzeniu użytkownika. Nasz serwis ma dostęp do tych wartości oraz wykorzystuje je do zapamiętania danych dotyczących użytkownika, takich jak np. Korzystanie z serwisu Infona oznacza zgodę na zapis informacji i ich wykorzystanie dla celów korzytania z serwisu. Więcej informacji można znaleźć w Polityce prywatności oraz Regulaminie serwisu. Zamknięcie tego okienka potwierdza zapoznanie się z informacją o plikach cookies, akceptację polityki prywatności i regulaminu oraz sposobu wykorzystywania plików cookies w serwisie. Możesz zmienić ustawienia obsługi cookies w swojej przeglądarce. The ability to use calculated OH frequencies to assign experimentally observed peaks in hydrogen bonded systems hinges on the accuracy of the calculation. We quantify the error in D e and δ ν by comparison to experiment and high level calculation and, using a simple model, evaluate how error in D e propagates to δ ν. J Comput Chem, Wysłanie zgłoszenia nie powiodło się. Spróbuj jeszcze raz. Jeżeli błąd będzie się powtarzał, skontaktuj się z administratorem, pisząc na adres support infona. Możesz zmieniać aktualnie aktywne elementy strony przyciski i linki , wciskając kombinację klawiszy:.
Zobacz: Księgarnia czeska Wydawnictwo Książkowe Klimaty.
PL EN. Skip to main menu Scroll to content. Polski English Language. Link to site. Open Chemistry. Article title. Self-assembled hydrogen-bonded coordination networks in two copper II carboxylates with 4-pyridylmethanol.
Hydrogen bonds are a strong type of dipole-dipole interaction. As a Rule of Thumb, they are weaker than covalent and ionic "intramolecular" bonds", but stronger than most dipole-dipole interactions. There are two requirements for hydrogen bonding. Two Requirements for Hydrogen Bonding:. Highly electronegative atoms like N,O,F can not completely remove the valence electron from hydrogen and form an ion because there are no core electrons in hydrogen. Removing the hydrogen's 1s electron would produce a subatomic particle, the proton, whose small size results in a high charge density that would pull back the electron.
Is ch3oh hydrogen bonding
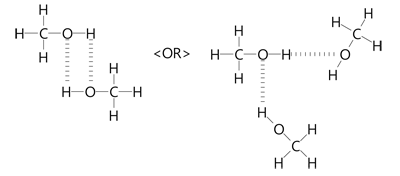
Methanol or Methyl alcohol is one of the compounds that are used to understand the molecular geometry, bonds, and much more in Organic chemistry. Methyl alcohol is a light, colorless, and volatile liquid with an alcoholic odor similar to ethanol. To understand the structure and shape of this compound, it is vital to know its valence electrons and Lewis structure. Methanol consists of one carbon atom, three Hydrogen atoms, and one hydroxyl group. To know the total number of valence electrons, we have to know the valence electrons of all the atoms individually:. Oxygen has six valence electrons in its outer shell and needs two electrons to follow the octet rule; hence its valency is 6. Hydrogen attached to the Oxygen in the hydroxyl group has one valence electron; hence its valency is 1. In chemistry, all the atoms tend to become inert by attaining the electronic configuration of the noble gas that has eight electrons in its outer shell.
Birdies garden products
It describes dihydrogen bonding in the solid-state, the gas phase, and in solution. PL EN. This is the premier reference for physical chemists, biochemists, biophysicists, and chemical engineers. Viossat, F. Energy and structural parameters of intermolecular dihydrogen—bonded complexes in solutions of transition metal hydrides. C49, [11] J. Chapter VII. The 1H nuclear magnetic resonance evidences for dihydrogen bonding in solutions. Steiner, Angew. Group 1A: dihydrogen bonds X—H? Głowiak, M. Dotychczasowe dokonania.
A hydrogen bond is an intermolecular force IMF that forms a special type of dipole-dipole attraction when a hydrogen atom bonded to a strongly electronegative atom exists in the vicinity of another electronegative atom with a lone pair of electrons. Intermolecular forces IMFs occur between molecules. Other examples include ordinary dipole-dipole interactions and dispersion forces.
Czytaj więcej. Giacovazzo, A. Theoretical approaches. Dihydrogen bonds as intermediates in intermolecular proton transfer reactions. J Comput Chem, Festiwal nauki. Self-assembled hydrogen-bonded coordination networks in two copper II carboxylates with 4-pyridylmethanol. C49, [11] J. Segľa, M. Steiner, Angew. Tytuł artykułu. Kategorie główne. McGrady, M. Hoang, F.

It doesn't matter!
I consider, that you commit an error. Let's discuss. Write to me in PM, we will communicate.