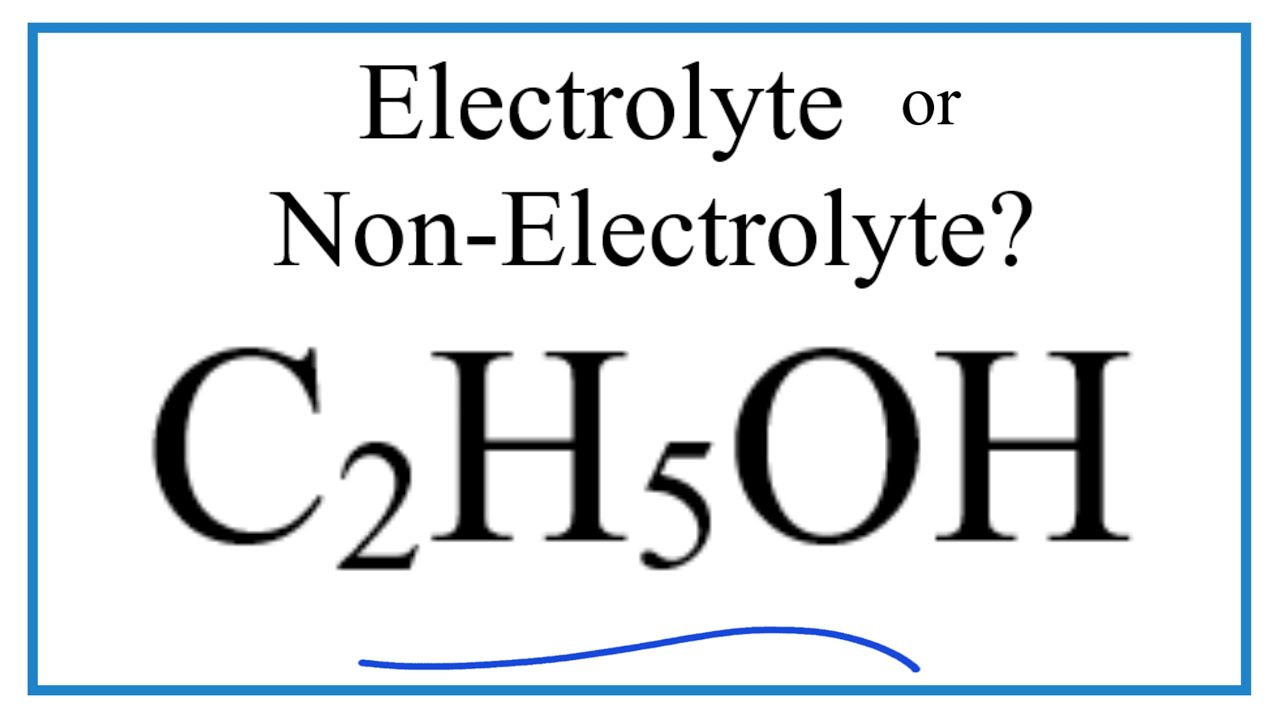
Is ch3ch2oh an electrolyte
No, ethanol C2H5OH is not an electrolyte. Ethanol does not dissociate into ions in water, so it does not conduct electricity and is not classified as an electrolyte. Well, this was just a simple answer.
Submitted by Ralph W. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. All of the compounds below dissolve in water. Which of them are strong electrolytes, which are weak electrolytes, and which are nonelectrolytes? Name two acids that are strong electrolytes and one acid that is a weak electrolyte.
Is ch3ch2oh an electrolyte
Submitted by Katrina D. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Define a strong electrolyte, weak electrolyte and nonelectrolyte. State if the following compounds are strong electrolytes, weak electrolytes or nonelectrolytes. Write chemical equations for the strong electrolytes and weak electrolyte in water. Which of the following compounds, based upon their chemical formulas, would you expect to be strong electrolytes? There can be more than one.
When ethanol is dissolved in water, it does not ionize. Ask unlimited questions and get video answers from our expert STEM educators. Yes,the Ethyl alcohol ethanol is an electrolyte.
Ethanol has been used in a variety of applications, from fuel to drinkable alcohol. One of these uses is as an electrolyte, a substance that conducts electrical current when dissolved in water. However, this use of ethanol is not particularly effective due to its low ionic content and instability in solution. When ethanol is dissolved in water, it does not produce many ions, which limits the amount of electrical current it can conduct. Additionally, ethanol quickly breaks down when mixed with water, further reducing its usefulness as an electrolyte. This means that while ethanol can be used as an electrolyte, it is not recommended due to its poor performance and high instability.
One of the most important properties of water is its ability to dissolve a wide variety of substances. Solutions in which water is the dissolving medium are called aqueous solutions. For electrolytes, water is the most important solvent. Ethanol, ammonia, and acetic acid are some of the non-aqueous solvents that are able to dissolve electrolytes. Substances that give ions when dissolved in water are called electrolytes. They can be divided into acids, bases, and salts, because they all give ions when dissolved in water.
Is ch3ch2oh an electrolyte
Car batteries are used around the world to provide the power to start car engines. One essential component of car batteries is the strong electrolyte sulfuric acid. In the battery, this material ionizes into hydrogen ions and sulfate ions. As the battery is used, the concentrations of these ions decreases. Older batteries had openings in the top where new sulfuric acid could be added to replenish the supply.
Jizztube
These minerals are essential for the body to function properly, and they play a key role in maintaining fluid balance, transmitting nerve impulses and muscle contraction. Zhenhong Qi. Glucose, ethanol and urea are all non-electrolytes. He has also worked as a radio reporter and holds a degree from Moody College of Communication. Log in. Is Reactivity a Physical or Chemical Property? Still have questions? Yan, J. Yes, rubbing alcohol is an electrolyte. Log in to watch this video No, ethanol is not an electrolyte. More answers. Is wax an electrolyte? However, this use of ethanol is not particularly effective due to its low ionic content and instability in solution.
Electrolytes were previously described as substances that yield ions when dissolved in water, which means that aqueous solutions of electrolytes are able to conduct electricity. It should be clear that soluble ionic compounds are electrolytes.
Suggested Textbook. In the case of ethanol, it exists as a covalent molecule with a molecular structure held together by covalent bonds. Is wax an electrolyte? Scroll to Top. Share Question Copy Link. The hydrogen-oxygen and carbon-oxygen bonds are polar covalent bonds. This is because the hydrogen atom has a higher electronegativity than the oxygen atom. Wax is not an electrolyte. It is not an electrolyte because it does not form ions when in a solution. Download Citation. Anything that hydrogen bond with the water molecules makes it water soluble, such as alcohols. Ne Neon : Neon is a noble gas and does not form ions in solution. Hint: Review what defines a strong electrolyte! Snapsolve any problem by taking a picture.

I apologise, but, in my opinion, you are not right. I suggest it to discuss. Write to me in PM.