Is ammonia a strong electrolyte
Ammonia is un-ionized in the gaseous state but in the aqueous solution is a weak electrolyte because of the following reason:. Byju's Answer. Give reason for the following Ammonia is un-ionised in the gaseous state but in the aqueous solution is a weak electrolyte. Open in App.
NH3 Ammonia is a weak electrolyte. Well, this was just a simple answer. But there are few more things to know about this topic which will make your concept super clear. Ammonia NH3 is considered a weak electrolyte because it only partially dissociates into ions when dissolved in water. In the context of electrolytes, substances can be classified into three categories based on their ability to conduct electricity when dissolved in a solvent usually water : 1.
Is ammonia a strong electrolyte
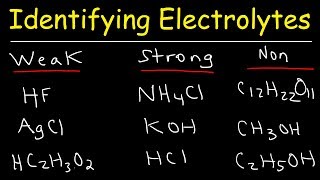
Electrolyte is a solution and a medium that consists of free ions which help in the conduction of electricity. The solute in an electrolyte will break up from its molecular form to form free ions. A strong electrolyte consists of a solute that dissociates into free ions in large quantity while a weak electrolyte does not release much of the free ions. Some of the examples of strong electrolyte are sodium nitrate, sodium chloride and sodium sulphate and one example for weak a electrolytes is ammonia solution. Weak electrolytes are solutions that have the substances dissolved in them in the form of molecules rather than ions. Ammonia in water is an example for weak electrolyte. It exists as molecule in water and to some extent get dissociated as ion. Since the weak electrolytes have fewer ions in the solution, it acts as weak conductor of electricity. The weak electrolyte consists of ions and molecules in equilibrium with each other. They exist as molecules as well as dissociate back into ions. The reactants molecular form and the products ionic form will be in equilibrium.
The dissociation of ammonia in water can be represented by the following equilibrium reaction: 5.
Cronk Syllabus Topics. Electrolytes musical accompaniment to this topic are substances that create ionic species in aqueous solution. We can demonstrate the existence of charge carriers in solution by means of a simple experiment. The conductivity of aqueous media can be observed by using a pair of electrodes, connected to a voltage source, that we immerse in the solution. The current the solution conducts then can be readily measured; we use a light bulb as a visual indicator of the conductivity of a solution.
One of the most important properties of water is its ability to dissolve a wide variety of substances. Solutions in which water is the dissolving medium are called aqueous solutions. For electrolytes, water is the most important solvent. Ethanol, ammonia, and acetic acid are some of the non-aqueous solvents that are able to dissolve electrolytes. Substances that give ions when dissolved in water are called electrolytes. They can be divided into acids, bases, and salts, because they all give ions when dissolved in water.
Is ammonia a strong electrolyte
Electrolytes are chemicals that break into ions ionize when they are dissolved in water. The positively-charged ions are called cations, while the negatively charged ions are called anions. Substances are categorized as strong electrolytes, weak electrolytes, or nonelectrolytes. Strong electrolytes completely ionize in water. However, it does not mean the chemical completely dissolves in water!
Series calculus 2 cheat sheet
Scroll to Top. In such a case, we say that sodium chloride is a strong electrolyte. However, when we perform our conductivity test with an acetic acid solution, we find that the light bulb glows, albeit rather weakly compared to the brightness observed for the sodium chloride solution. Log in. Follow Us :. Let us represent what we think is going on with these contrasting cases of the dissolution of a molecular and an ionic compound by writing the following chemical equations:. Q: Is ammonia an electrolyte or non electrolyte? Study now See answer 1. Well, this was just a simple answer. The ions are free to diffuse individually in a homogeneous mixture, and when a voltage is applied, the ions will move according to the electric potential energy difference between electrodes, thus carrying electric current. Cronk Syllabus Topics. The equation given below shows the dissociation of ammonia into ions and vice versa. Accordingly, we classify acetic acid as a weak acid. Byju's Answer.
Electrolytes are chemicals that break into ions in water. Aqueous solutions containing electrolytes conduct electricity. Strong electrolytes include the strong acids , strong bases , and salts.
Its a non electrolyte. It exists as molecule in water and to some extent get dissociated as ion. Schedule Topics. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. Examples include ionic compounds like NaCl sodium chloride or strong acids like HCl hydrochloric acid. It forms ammonium hydroxide NH4OH , which is a base, and basic solutions are electrolytic. Ammonia in water is an electrolyte. Scroll to Top. The dissociation of ammonia in water can be represented by the following equilibrium reaction: 5. Write your answer This reaction of a solute in aqueous solution gives rise to chemically distinct products.

In my opinion you have deceived, as child.