Ionic compounds are soluble in water
The sugar we use to sweeten coffee or tea is a molecular solidin which the individual molecules are held together by relatively weak intermolecular forces. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these C 12 H 22 O 11 molecules are released into solution.
A subscription to JoVE is required to view this content. We recommend downloading the newest version of Flash here, but we support all versions 10 and above. If that doesn't help, please let us know. Unable to load video. Please check your Internet connection and reload this page.
Ionic compounds are soluble in water
We have learned that solutions can be formed in a variety of combinations using solids, liquids, and gases. We also know that solutions have constant composition, and that this composition can be varied up to a point to maintain the homogeneous nature of the solution. But how exactly do solutions form? Why is it that oil and water will not form a solution, and yet vinegar and water will? Why could we dissolve table salt in water, but not in vegetable oil? The reasons why solutions will form will be explored in this section, along with a discussion of why water is used most frequently to dissolve substances of various types. In most cases, only a certain maximum amount of solute can be dissolved in a given amount of solvent. This maximum amount is specified as the solubility of the solute. It is usually expressed in terms of the amount of solute that can dissolve in g of the solvent at a given temperature. These solubilities vary widely. NaCl can dissolve up to
A JoVE representative will be in touch with you shortly. Discuss the idea of water as the "universal solvent".
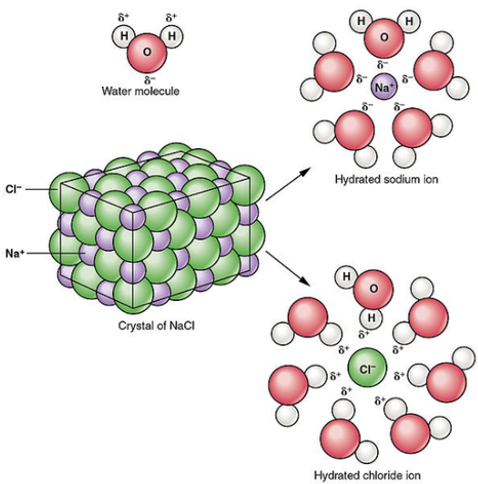
To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge. When you place an ionic substance in water, the water molecules attract the positive and negative ions from the crystal. The positive ions have several water molecules around them, all with their "O" atoms close to the positive ion. The negative ions have several water molecules around them, all with their "H" atoms close to the negative ion.
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte. Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance.
Ionic compounds are soluble in water
The extent to which a substance may be dissolved in water, or any solvent, is quantitatively expressed as its solubility , defined as the maximum concentration of a substance that can be achieved under specified conditions. Substances with relatively large solubilities are said to be soluble. A substance will precipitate when solution conditions are such that its concentration exceeds its solubility. Substances with relatively low solubilities are said to be insoluble , and these are the substances that readily precipitate from solution. More information on these important concepts is provided in the text chapter on solutions. Therefore, Pb NO 3 2 is soluble. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. Not all ionic compounds are able soluble in water.
Imdb atomic blonde
It takes energy to break the bonds between the C 12 H 22 O 11 molecules in sucrose. Thus, most of the compound remains undissolved in water. Create Account. Electrolyte Solutions: Dissolved Ionic Solids When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. R-Rahaman Raza. Slightly soluble salts give solutions that fall between these extremes. A solution of Unable to load video. Outside of this cluster of spheres are seventeen clusters of three spheres, which include one red and two white spheres. A more complex water-soluble ionic compound like sodium nitrate contains ions that are composed of multiple atoms covalently bound together, or polyatomic ions. The amount of salt that must be added to a given volume of solvent to form a saturated solution is called the solubility of the salt. Immiscible - Liquids that do not have the ability to dissolve in each other. As a result, the ions stay intact and do not separate. Reset Password.
Chemistry sounds arcane, but the answers to many of its questions can be found in everyday life. When we ponder whether ionic compounds are soluble in water, we can simply think about table salt. Table salt is sodium chloride , an ionic compound comprising sodium cations and chloride anions.
Question 67e Silver chloride AgCl is an example of an insoluble salt. Continue Learn more Close. What are ionic compounds? As a result, the ions stay intact and do not separate. Chapter Chemical Kinetics. You have already requested a trial and a JoVE representative will be in touch with you shortly. Question cd Explanation: To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. In most cases, only a certain maximum amount of solute can be dissolved in a given amount of solvent.

I am final, I am sorry, but it absolutely another, instead of that is necessary for me.
Thanks for an explanation. All ingenious is simple.