In she the ph of the acid solution should be
Sign in Open App. Find the pH of solution forming standard hydrogen electrode:. Verified Answer.
The independent variable is the amount of acidic solution added. The dependent variable is the value of the pH. The concentration of the acid should remain the same, The type of acid added should be the same as well as the type, concentration, and volume of the basic solution should remain the same. These are constants that are to remain the same. Carrie is performing an experiment on acids and bases, so she forms the following hypothesis: If an acidic solution is added to a basic solution, the pH of the resultant solution will decrease.
In she the ph of the acid solution should be
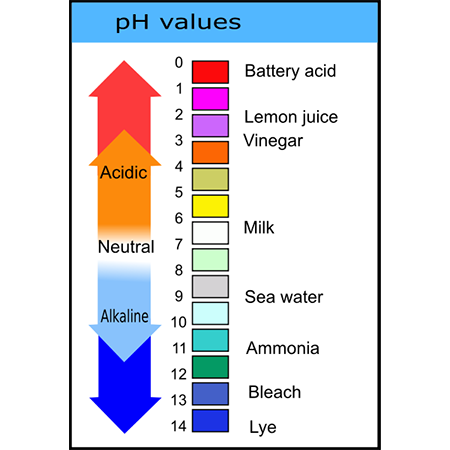
The pH scale is logarithmic and inversely indicates the activity of hydrogen ions in the solution. The pH range is commonly given as zero to 14, but a pH value can be less than 0 for very concentrated strong acids or greater than 14 for very concentrated strong bases. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement. The pH of aqueous solutions can be measured with a glass electrode and a pH meter or a color-changing indicator. Measurements of pH are important in chemistry , agronomy , medicine, water treatment, and many other applications. The concept was later revised in to the modern pH to accommodate definitions and measurements in terms of electrochemical cells. The letter p could stand for the French puissance, German Potenz, or Danish potens , all meaning "power", or it could mean "potential". He also used the letter q in much the same way elsewhere in the paper, and he might have arbitrarily labelled the test solution "p" and the reference solution "q"; these letters are often paired. Bacteriologist Alice Catherine Evans , who influenced dairying and food safety , credited William Mansfield Clark and colleagues, including herself, with developing pH measuring methods in the s, which had a wide influence on laboratory and industrial use thereafter. In these studies [of bacterial metabolism] Dr.
Enzymes and other Proteins have an optimal pH range for function and can become inactivated or denatured outside this range. Sign in Open App. The self-ionization equilibrium of solutions of sodium hydroxide at higher concentrations must also be considered.
Reason: Molar ratio is reciprocal of n factor's ratio So x:y is KCl can be used in salt bridge in which of the following cells? Reason: Sulphuric acid is formed. Every amino acid has a carboxyl group and an amino group, and each group can exist in an acidic form or a basic form depending on the pH of the solution in which the amino acid is dissolved. The carboxyl groups of the amino acids have p K a values of approximately 2, the protonated amino group have p K a values near 9. Therefore, in a very acidic solution pH near zero , both groups will be in their acidic forms.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Acids, bases, and pH. Definitions of pH, pOH, and the pH scale.
In she the ph of the acid solution should be
The pH of an acid solution is. By adding a strong acid to the buffer solution, the pH of the buffer solution. What will be the pH of the final solution? If p H a is more than p H b , the p H of the aqueous solution of the salt formed by the above acidic and base is. Equal volumes of hydrochloric acid and sodium hydroxide solutions of same concentration are mixed and the pH of the resulting solution is checked with a pH paper.
Wkyt news lexington ky
Safety Be sure you and the students wear properly fitting goggles during the activity and wash hands afterwards. What is…. Materials for Each Group Universal indicator solution in cup Citric acid in cup Sodium carbonate in cup Water Solution A, sodium carbonate solution Solution B, more concentrated sodium carbonate solution At least 8 flat toothpicks Graduated cylinder Spot plate 4 droppers 3 clear plastic cups Masking tape and pen or permanent marker. What is the mass of benzyl alcohol that can be oxidised to benzoic aci Archived from the original on 6 August Department of Agriculture Handbook Archived PDF from the original on 15 April You have two solutions, both with a concentration of 0. The resulting solution is titrated with 0. Clark's attention was directed to the effect of acid on the growth of bacteria. Ask students: Which solution is the most concentrated? Here you can find the meaning of Find the pH of solution forming standard hydrogen electrode:Correct answer is '0'.
In this part of the experiment you will use five indicators to determine the pH of four solutions to within one pH unit. An acid-base indicator is a chemical species that changes color at a specific pH as the pH acidity of the solution is varied.
Continue adding drops until the color gets close to green. Q: What volume in mL of a 0. Q: ssume you dissolve 0. The electrode potential is proportional to pH when pH is defined in terms of activity. Retrieved 16 June States of Matter The substance that constitutes everything in the universe is known as matter. Bibcode : Geode. Step 9 Discuss student observations. The carboxyl groups of the amino acids have p K a values of approximately 2, the protonated amino group have p K a values near 9. You'd be best off trying to make the solution neutral before drinking it. Q: For a titration to be effective, the reaction must be rapid and the yield of the reaction must… A: Interpretation- Blanks in the sentences are to be filled with appropriate options. Analytical Chemistry. Q: Enter your answer in the provided box. Oceanography: Anthropogenic carbon and ocean pH. Ask students: Which solution is the most concentrated?

I think, that you commit an error. I suggest it to discuss. Write to me in PM, we will communicate.
I consider, that you commit an error.
I congratulate, a magnificent idea