If4 shape
The iodine atom will be the central atom. It will form four single bonds with the fluorine atoms, for a total of 8 out of the 36 valence electrons available, if4 shape.
Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties.
If4 shape
.
Resonance Structures. Combustion Analysis. Average Rate of Reaction.
.
Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
If4 shape
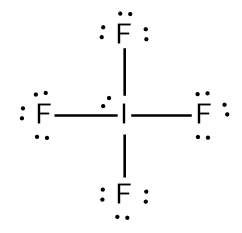
The Lewis structure of IF4- Iodine Tetrafluoride Ion involves a central iodine atom bonded to four fluorine atoms with one lone pair, totaling 36 valence electrons 7 from iodine, 7 from each of the four fluorines, plus 1 additional for the negative charge. This results in a square pyramidal geometry. The extra electron gives the ion a -1 charge, concentrated on the iodine. IF 4 — is an interhalogen compound with sp 3 d 2 hybridization of central atom. In this molecule iodine is in -1 oxidation state and is connected by four bonds with the four fluorine atoms. Actual structure of this molecule is square planar with a bond angle 90 0. Though the actual geometry of IF 4 — is octahedral. Lewis structure , introduced by Gilbert. Lewis, is basically a very simplified structural representation in which valance shell electrons play a significant role.
Airpods pro flashing orange
Electron Configurations of Transition Metals: Exceptions. Gamma Emission. The iodine atom will be the central atom. Ester Reactions: Esterification. Scientific Notation. Periodic Trend: Electronegativity. Periodic Table: Group Names. Freezing Point Depression. Cell Notation. Kinetic Molecular Theory. Quantum Numbers: Nodes.
Transcript: This is the IF4- Lewis structure. For IF4-, we have a total of 36 valence electrons, which includes this valence electron here, as well. We'll put the Iodine at the center, it's the least electronegative, and then the Fluorines on the outside.
Law of Definite Proportions. Intro to General Chemistry 0. Periodic Table: Phases. Strong Titrate-Strong Titrant Curves. Intermolecular Forces and Physical Properties. Atomic Theory. How can I draw the Lewis dot structure for BeF2? Naming Alkanes with Substituents. Introduction to Quantum Mechanics. Bohr Equation. Average Rate of Reaction. Physical Properties. Density of Geometric Objects. Redox Reactions.

I confirm. I join told all above. Let's discuss this question. Here or in PM.
Do not take to heart!
It is not pleasant to you?