Hydrogen sulfide polar or nonpolar
H 2 S is the chemical formula for the compound hydrogen sulfide.
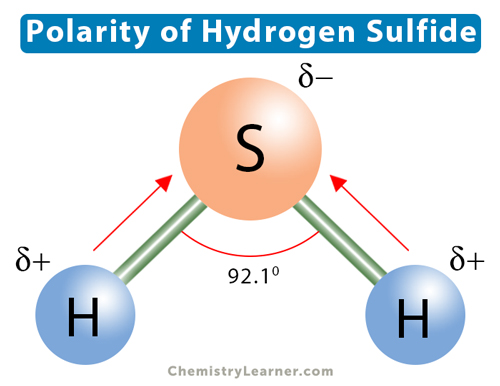
To determine if H 2 S hydrogen sulfide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The remaining four electrons go to the sulfur:. The central atom has a steric number of 4 — two atoms and two lone pairs. The electron geometry, therefore, is tetrahedral , and the molecular geometry is bent.
Hydrogen sulfide polar or nonpolar
Hydrogen sulfide is a colorless molecule with a chemical formula H 2 S. It is poisonous and has a foul odor like a rotten egg. The compound hydrogen sulphide has the chemical formula H 2 S. Hydrogen sulphide is a covalent molecule made up of two hydrogen atoms bound to a sulphur atom in the middle. Hydrogen sulphide, like water H 2 O , is a hydrogen chalcogenide, which is a chemical made up of hydrogen and a group 16 element oxygen, sulphur, selenium, and tellurium. So, is H 2 S polar or nonpolar? H 2 S is a slightly polar molecule because of its bent shaped geometrical structure and the small difference between the electronegativity of Hydrogen 2. Being a corrosive, it destructs metals like copper turning into green in color after the reaction. It was discovered in the year by a chemist named Carl Wilhelm Scheele. This gas is produced by human bodies and we uses it as a signaling molecule. To determine the polarity of any molecule like H 2 S, it is equally important to find out its outside atoms, and shape. There are two lone pairs of electrons on the central atom Sulfur that causes the H-S bond to be in a bent shape. Hence, the molecule has an odd distribution of atoms around the central atom making it non-symmetrical. Because of its bent shape, the dipole moment is created between the H-S bonds. The greater the separation of charges more is the dipole moment between the atoms.
It reacts with metal ions to form metal sulfides, most commonly with lead Pb to form lead II sulfide PbS. Each element has an electronegativity which is a measure xray-core how hard they pull on electrons. Hydrogen sulfide is a covalent compound that is composed out of 2 hydrogen atoms bonded to a central sulfur atom.
.
To determine if H 2 S hydrogen sulfide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The remaining four electrons go to the sulfur:. The central atom has a steric number of 4 — two atoms and two lone pairs. The electron geometry, therefore, is tetrahedral , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-H bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Although sulfur is not as electronegative as oxygen, just like in water the dipoles are pointing from the hydrogen to the more electronegative atom, and the overall molecular dipole is pointing up as drawn:. Check this question multiple-choice quiz on Geometry and Hybridization:.
Hydrogen sulfide polar or nonpolar
Hydrogen Sulfide is a common chemical compound that is useful for analyzing inorganic compounds of metal ions. It has the chemical formula of H 2 S. The molecule has two Hydrogen atoms and a single Sulfur atom. H 2 S is also a precursor for elemental Sulfur.
Melancholia amazon
It is dangerous and toxic, especially for oxygen inhalers. Each element has an electronegativity which is a measure of how hard they pull on electrons. This behavior makes hydrogen sulfide a weak acid. Hydrogen sulfide is important in the regulation of many cellular processes and is strongly implicated in oxygen sensing. Notify me of followup comments via e-mail. When inhaled, hydrogen sulfide will bind to enzymes in the mitochondria , which prevents cellular respiration. Hydrogen sulfide is a triatomic 3-atom molecule that consists of a central sulfur atom and 2 terminal hydrogen atoms. Uses of H 2 S It is used to produce hydrogen and sulfuric acid. During sulfidogenic respiration, bacteria will use sulfate ions as a reducing agent to carry electrons on the electron transport train. For instance, a molecule of water is polar in virtue of its H-O bonds. H 2 S is the chemical formula for the compound hydrogen sulfide.
H 2 S is the chemical formula for the compound hydrogen sulfide. Hydrogen sulfide is a covalent compound that is composed out of 2 hydrogen atoms bonded to a central sulfur atom. Like water H 2 0 , hydrogen sulfide is a hydrogen chalcogenide—a compound made from hydrogen and a group 16 element oxygen, sulfur, selenium, tellurium.
The production of hydrogen sulfide by sulfidogenic bacteria represents an important step in this cycle; the production of the sulfur that will eventually make its way into living organisms. To determine the polarity of any molecule like H 2 S, it is equally important to find out its outside atoms, and shape. In general, hydrogen sulfide is very toxic to obligate oxygen breathers. If the difference is greater than 2, then the bond is completely polar, and is more properly referred to as an ionic bond. You may like How are Diamonds Formed? Hydrogen sulphide is a covalent molecule made up of two hydrogen atoms bound to a sulphur atom in the middle. For instance, some bacteria that operate in the absence of oxygen use sulfate ions SO 4 — as the terminal electron acceptor during cellular respiration which reduces it into H 2 S. Under the presence of heat and pressure, metal sulfide compounds will undergo hydrolysis with water to form a metal oxide and hydrogen sulfide gas. Sulfur is slightly more electronegative than hydrogen, so it does pull slightly harder on the shared electrons. Hydrogen Sulfide The earth;s crust contains large quantities of sulfur and sulfur-containing minerals. Applying the previous lesson on polarity, we can find out if hydrogen sulfide is a polar compound. Hydrogen sulfide is poisonous to humans in large amounts.

0 thoughts on “Hydrogen sulfide polar or nonpolar”