Hybridization of carbon in co2
In this article, we will delve into the intriguing world of chemistry to explore the hybridization of CO 2. Carbon dioxide is an interesting molecule, with carbon at its core exhibiting sp hybridization. This hybridization arises due to the carbon atom being bonded to two other atoms, hybridization of carbon in co2, which can be either two double bonds or a combination of one single and one triple bond.
The carbon dioxide or CO2 has sp type hybridisation. This type of hybridisation occurs as an outcome of the carbon being bound to two different atoms. The atom of the carbon comprises 2 double bonds, i. However, this is not sufficient for creating bonds involving the oxygen. Therefore, one electron from the 2s orbital shifts from the 2s level to 2p level, which leads to the creation of 2 hybrid orbitals.
Hybridization of carbon in co2
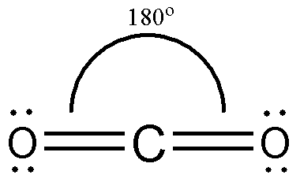
We will learn about the hybridization of CO 2 on this page. Carbon dioxide basically has a sp hybridization type. This type of hybridization occurs as a result of carbon being bound to two other atoms. We can determine this by closely observing each atom of CO 2. In determining the hybridization of carbon dioxide, we will take the carbon atom first. The carbon atom has two effective pairs or two double bonds exist in it. However, this is not enough to form bonds with oxygen. What happens next is that one electron from 2s orbital moves from the 2s level to 2p level which results in the formation of two hybrid orbitals. Now, these sp hybridized orbitals of the carbon atom overlap with two p orbitals of the oxygen atoms to form 2 sigma bonds. As for the two remaining p electrons they will be used to form a pi bond. In carbon dioxide molecule, oxygen also hybridizes its orbitals to form three sp 2 hybrid orbitals. The p orbital in oxygen remains unchanged and is mainly used to form a pi bond. However, out of the three sp hybrid orbitals, only one will be used to form a bond with the carbon atom. CO 2 molecular geometry is based on a linear arrangement. This geometric shape is mainly due to the presence of a sigma bond and valence electron pairs repelling each other where they are forced to move to the opposite side of the carbon atom.
The absence of the double bonds represents a hybridisation of sp3. In the case of carbon dioxide, carbon is the atom in the centre.
To determine the hybridization of carbon dioxide, let us take the carbon atom first. The carbon atom has two double bonds, or two effective pairs exist in it. However, this is not enough to produce bonds with oxygen. So, then, one electron from 2s orbital moves from the 2s level to the 2p level that results in the formation of two hybrid orbitals. Now, these hybridized sp orbitals of carbon atoms overlap with two p orbitals of the oxygen atoms to produce 2 sigma bonds. They are used to form a pi bond as for the two remaining p electrons.
Welcome to a comprehensive exploration of the carbon dioxide molecule or CO2. Lewis structures, also known as electron dot diagrams, were introduced by Gilbert N. Lewis in They play a crucial role in visualizing the arrangement of valence electrons among atoms in a molecule, helping us predict its physical and chemical properties. A Lewis structure is a type of shorthand notation that scientists use to describe the distribution of electrons in molecules. Lewis structures are a foundational tool in chemistry, allowing us to visualize how atoms share or transfer electrons to form molecules. When creating the Lewis structure for carbon dioxide, we start with the central carbon atom, which forms double bonds with two oxygen atoms on either side.
Hybridization of carbon in co2
To determine the hybridization of carbon dioxide, let us take the carbon atom first. The carbon atom has two double bonds, or two effective pairs exist in it. However, this is not enough to produce bonds with oxygen. So, then, one electron from 2s orbital moves from the 2s level to the 2p level that results in the formation of two hybrid orbitals. Now, these hybridized sp orbitals of carbon atoms overlap with two p orbitals of the oxygen atoms to produce 2 sigma bonds.
Close rhyming words
An atom with 2 or more than 2 double bonds, or with a single-triple bond, comprises hybridisation of sp. However, out of the three sp hybrid orbitals, only one will be used to form a bond with the carbon atom. JEE Marking Scheme. Band Theory. Out of the three sp hybrid orbitals, only one is used to form a bond with the carbon atom. Then, we can complete the octets on the outer shell. It forms three sp 2 hybrid orbitals. Each of the 2p orbital, 2px 2py, 2pz now holds one electron. The carbon atom has two effective pairs or two double bonds exist in it. Lastly, if it is bonded with two atoms, then it is sp. The formation of CO 2 consists of two particles: Oxygen and Carbon. As we can see, Oxygen has 8 electrons, which is perfect. Finally, we have completed the formation of an octet.
In this article, we will delve into the intriguing world of chemistry to explore the hybridization of CO 2.
Allotment of Examination Centre. Carbon has 6 electrons, whereas Oxygen has 8 electrons. More Articles for Chemistry. Here, if it is bonded with four atoms, then it is Learn more. One electron from the 2s orbital hops to the 2p level, leading to the creation of two hybrid orbitals. Every single oxygen atom in the carbon dioxide has a single double bond with the carbon. Oxalic-Acid vs KMnO4. Test Series. In CO2, carbon is the atom in the centre. Band Theory. As we can see, Oxygen has 8 electrons, which is perfect. These sp hybridized orbitals of the carbon atom then overlap with the p orbitals of the oxygen atoms, forming 2 sigma bonds. Carbon dioxide basically has a sp hybridization type.

You are not right. I am assured. I can prove it. Write to me in PM.
Amusing state of affairs