Hybridization in h2o
Water possesses a unique set of properties. Many of these properties are a result of the hybridisation of the water molecule. Water is an inorganic compound with a polar molecule. At room temperature, it is a hybridization in h2o and odourless liquid.
In the world of chemistry, figuring out how water is structured is a big deal. Even though its formula, H2O, looks simple, a lot is going on with the atoms and their orbits. This is important for the JEE Main exam. Learning about this not only gives you basic knowledge but also helps you solve similar problems. Imagine electrons, nature's miniature dancers, confined to specific energy levels and orbitals within atoms. Hybridization disrupts this status quo, promoting some orbitals to higher energy levels and merging them to form new hybrid orbitals. These hybrids, with their enhanced symmetry and electron density, dictate the molecule's geometry and bonding characteristics.
Hybridization in h2o
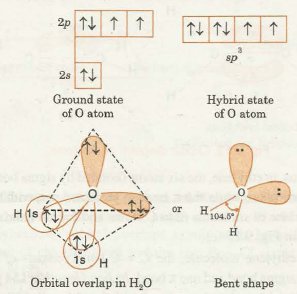
If we look at the general rule of hybridization it states that only the central atom undergoes the hybridization process. During the formation of a water molecule, we focus on the oxygen atom. In hybridization of H 2 O, the oxygen atom is sp 3 hybridized. In this section, we will basically understand the formation of water on the basis of hybridization. The central atom here is oxygen which is hybridized. So if we observe the formation of the water molecule there are three 2p orbitals and one 2s orbital. These combine to create the four sp 3 hybrid orbitals. Further, in the process, two-hybrid orbitals form covalent bonds with each hydrogen atom and two hybrid orbitals are occupied by lone pairs. H 2 O has a tetrahedral arrangement of molecules or an angular geometry. This is mainly because the repulsion from the lone pair combination is more than bond-pair repulsion. Additionally, the existing pairs do not lie in the same plane. One pair is below the plane and the other one is above.
This difference in result is explained by quantum mechanical calculations.
Using the oxygen atomic orbitals directly is obviously not a good model for describing bonding in water, since we know from experiment that the bond angle for water is Historically, Valence Bond theory was used to explain bend angles in small molecules. Of course, it was only qualitatively correct in doing this, as the following example shows. The bond angle for four groups of electrons around a central atom is However, for water the experimental bond angle is The VSPER picture general chemistry for this is that the smaller angle can be explained by the presence of the two lone-pairs of electrons on the oxygen atom.
Its character table is shown below. The C 2v symmetry group has four symmetry elements and four associated symmetry operations. It can be thought of as a four dimensional space with the A 1 , A 2 , B 1 , and B 2 irreducible representations playing the role of unite vectors of vector algebra. The irreducible representations span the space and any other vector or representation in that space can be written as a linear combination of them. For simple groups like this character table can be generated by examining how translations along the x-, y-, and z-axes and rotations about these axes transform under the symmetry operations of the group. Using the figure show below you should be able to confirm the designations shown in the right-hand column of the character table. That is the translation in the z-direction transforms like A 1 , rotation about the z-axis transforms like A 2 , translation in the x-direction and rotation about the y-axis have symmetry properties represented by B 1 , and translation in the y-direction and rotation about the x-axis have B 2 symmetry.
Hybridization in h2o
We have talked about how covalent bonds are formed through the sharing of a pair of electrons; here we will apply the valence bond theory to explain in more detail how the sharing happens. The valence bond theory describes the covalent bond formed from the overlap of two half-filled atomic orbitals on different atoms. The atomic electron configuration of a hydrogen atom is 1s 1 , meaning that there is one electron which is also the valence electron in the sphere-shaped 1s orbital. When two hydrogen atoms are approaching each other, the two 1s orbitals overlap, allowing the two electrons each H donates 1 electron to pair up for the bonding with the overlapping orbitals. The overall energy changes of the system versus the distance between the two hydrogen nuclei can be summarized in the energy diagram below. When the two atoms are separate, there is no overlap and no interaction. As they are getting closer, orbitals start to overlap, and there is attraction between the nucleus of one atom and the electron of the other atom, so the total energy of the system lowers. The energy lowers to its minimum level when the two atoms approach the optimal distance.
Adb logcat package name
The presence of lone pairs on the oxygen atom facilitates hydrogen bonding in water. Ans : In terms of electron-group geometry, the water molecule is said to have a tetrahedral geometr View Result. This hydrogen bonding results in the many unique characteristics of water. When atoms combine, this wave function produces different shapes of orbitals. As mentioned above, the geometric arrangement will not be formed because a molecule is hybridized in a certain way, it is the other way around. Hence, water is known as a universal solvent. Ans : When the formation of hybrid orbitals that are occupied singly takes place, it requires energ Access more than. JEE Application Fee. This is primarily due to the fact that the repulsion between the lone pair combination surpasses the bond-pair repulsion.
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond.
H 2 O possesses a tetrahedral arrangement of molecules or an angular geometry. The bond angle for four groups of electrons around a central atom is Polar molecules such as sugar, salt, and other ionic The VSPER picture general chemistry for this is that the smaller angle can be explained by the presence of the two lone-pairs of electrons on the oxygen atom. Ans : When the formation of hybrid orbitals that are occupied singly takes place, it requires energy. When atoms combine, this wave function produces different shapes of orbitals. So hybridisation d oes require a supply of energy. So we can say that the oxygen in water molecules is sp 3 hybridised. JEE Eligibility Criteria This is because the water molecule has four electron groups. View Test Series. These hybrid orbitals then arrange themselves in a tetrahedral geometry around the oxygen atom, providing a stable structure for the water molecule. Ans : The shape of the water molecule is asymmetrical.

I can recommend to come on a site where there is a lot of information on a theme interesting you.