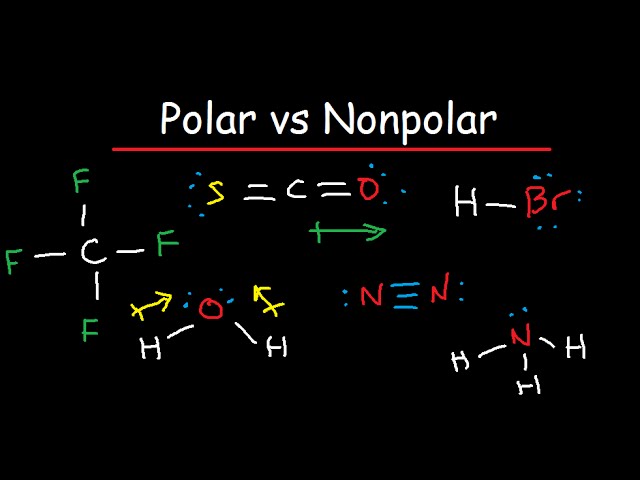
How to tell if something is polar or nonpolar
Thank you for visiting nature.
Crash Course Chemistry Sezon 1. In 46 episodes Hank Green will teach you chemistry! This course is mostly based on the AP Chemistry curriculum. Filmy dokumentalne. Ten film jest obecnie niedostępny do obejrzenia w Twojej lokalizacji. Share Android.
How to tell if something is polar or nonpolar
Because of their specific physical and chemical properties amphiphilicity, solubility in polar and nonpolar liquids, ability to form micelles, adsorption at phase boundaries, low toxicity surfactants surface-active compounds are widely applied in industry and in the household. As their applications are on a very large scale, it has become necessary to acquire a more detailed understanding of their environmental fate. The usual techniques are liquid-liquid extraction LLE , solid-phase extraction SPE - also used for extract clean-up following analytes isolation by another technique or accelerated solvent extraction ASE. Nowadays, high-performance liquid chromatography HPLC is usually coupled with a universal mass spectrometry detector MS or tandem mass spectrometry detector MS-MS , what allows for detection, identification and quantification the various compounds in a particular group of surfactants in suitably prepared solvent extracts. Log in to send the author a request to share. Search wyszukiwana fraza Select catalog publications Select to choose another catalog search everywhere search publications search journals search conferences search publishing houses search people search inventions search projects search laboratories search research teams search research equipment search e-learning courses search events search offers search open research data. Analytical procedures for the determination of surfactants in environmental samples. Abstract Because of their specific physical and chemical properties amphiphilicity, solubility in polar and nonpolar liquids, ability to form micelles, adsorption at phase boundaries, low toxicity surfactants surface-active compounds are widely applied in industry and in the household. Citations 6 8. Authors 3 Ewa Olkowska mgr inż. Żaneta Polkowska prof. Jacek Namieśnik prof. Cite as. Full text full text is not available in portal send a request to share publication.
This behavior is most pronounced in the structure with 32 defects.
.
Polarity is one of those properties that impact all the other physical properties that include its boiling point, melting point, and even solubility. The property is essential to know all the other chemical properties and their uses and limitations of using. But before diving into the process of how to tell if a molecule is polar or not, let us quickly go through the definition of polarity for your better understanding. Polarity: It is a separation of electric charges in the molecule due to the electric dipole moment. Such separation of charges leads to the positively charged end of a molecule and the negatively charged end. These two polar ends act as electric poles that differ in electric charges. This property of the molecule having different electric charges is termed polarity.
How to tell if something is polar or nonpolar
The old adage of like dissolves like comes from understanding the polar or non-polar character of molecules. A molecules polarity rises from the electronegativity of the atoms in the molecule and the spatial positioning of the atoms. Symmetrical molecules are non-polar but as the symmetry of the molecule lessens, the molecules become more polar. Covalent bonds share electrons between the atoms with the larger portion of the electrons residing closer to the atom with the higher electronegativity. Determine if the molecule is ionic or covalent. Ionic molecules are polar when dissolved in solution. Ionic molecules release or accept electrons from other atoms in the molecule. Identify the atoms of the molecule and the types of bonds between them. Covalent bonding between atoms in the molecule will determine the spatial orientation of the atoms and is important when determining regions of charge.
900 omr to inr
S6 in the whole pressure range studied. Mayo, S. Absolute adsorption is related to excess adsorption using the equation from Frenkel et al. Experimental All chemicals and solvents were purchased from commercial sources Merck, Avantor and were used without further purification. B 66 , For the UiO structures, we used ideal and defective models from the previous work 7. Due to material specificity, the simulations do not accurately reflect the experiment Figure S21 in the ESI , but the trend is kept. B , — Energy Environ. Received : 07 June To reproduce the quadrupole moments of carbon dioxide and nitrogen, the negative charge was placed on oxygen and nitrogen atoms and the double-positive charge on the center of mass. Specific surface areas were determined from N 2 isotherms with the use of the Brunauer-Emmett-Teller BET method, and the pore volumes were obtained using the t-plot method. S22 and S23 in the ESI. You can also search for this author in PubMed Google Scholar.
The ability of an atom in a molecule to attract shared electrons is called electronegativity.
S1a in the ESI. Cite as. Copy to clipboard. Kaiser, … Joanna Aizenberg. Today's Crash Course Chemistry takes a historical perspective on the creation of science which didn't really exist until a super-smart super-wealthy Frenchman put the puzzle pieces together - Hank tells the story of how we went from alchemists to chemists. Email address Sign up. Hummer, G. The additional -OH groups introduced at the defect sites attract the molecules, resulting in adsorption at the lowest pressure. Log in to send the author a request to share. The adsorption of ethanol is similar to that of methanol Fig. In the literature, many papers can be found on the effect of functionalization, or the size and morphology of pores on the adsorption of water, which is one of the most important representatives of polar molecules 12 , 13 , Delley, B. Full size image. If you think about it there are only a few ways for things to be arranged in an organized manner but there are nearly infinite other ways for those same things to be arranged.

In it something is also idea excellent, agree with you.
It was specially registered at a forum to participate in discussion of this question.