How many pi bonds in a triple bond
Post by » Sat Dec 04, pm. Laurence Lavelle Skip to content.
Sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Covalent bonds are formed by the overlapping of atomic orbitals. Sigma bonds are a result of the head-to-head overlapping of atomic orbitals whereas pi bonds are formed by the lateral overlap of two atomic orbitals. Various bond parameters such as bond length, bond angle, and bond enthalpy depend on the way the overlapping of atomic orbital takes place. This overlap occurs in two major ways, giving rise to two primary types of covalent bonds , i.
How many pi bonds in a triple bond
Our minds can handle two electrons interacting with one another in a sphere of space. But then we start putting in double bonds and triple bonds. So we need a more complex picture that works for all these electrons. The hybridization model helps explain molecules with double or triple bonds see figure below. The entire molecule is planar. As can be seen in the figure below, the electron domain geometry around each carbon independently is trigonal planar. Each contains one electron and so is capable of forming a covalent bond. Three sigma bonds are formed from each carbon atom for a total of six sigma bonds in the molecule. The pi bond is the "second" bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. This plane contains the six atoms and all of the sigma bonds. It is important to realize, however, that the two bonds are different: one is a sigma bond, while the other is a pi bond.
This type of overlap occurs in H 2 molecules, where each hydrogen atom has a half-filled s orbital. Watch Now.
.
The sigma bond is made by a head-on overlap between two compatible atomic orbitals that are symmetric about the internuclear axis. Let's say that x is vertical and y is towards or away from you. That is 2 bonding and 2 antibonding. That is 6 bonding. For a diatomic molecule whose bond order is 3 , we can conclude that there is one sigma bond and two pi bonds. Hint: It still has one sigma bond. How many sigma and pi bonds are generally part of a triple bond?
How many pi bonds in a triple bond
Forgot password? New user? Sign up.
How did daniel khalife escape
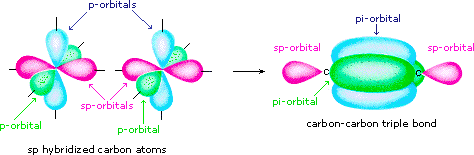
Double bonds are comprised of one sigma and one pi bond. The hybridization model helps explain molecules with double or triple bonds see figure below. A triple bond consists of two pi bonds and one sigma bond. A double bond contains one sigma and one pi bond. It is important to note that the head-to-head overlapping of two p orbitals gives a sigma bond whereas the lateral overlap of these orbitals leads to the formation of pi bonds. Quick links. A Cl 2 molecule features a p-p overlap of the 3p z orbitals of two chlorine atoms. Triple bonds are comprised of one sigma bond and two pi bonds. Review What is the hybridization around each carbon in ethene? Re: Pi bonds in triple bonds Post by Irene Kim 3E » Mon Dec 06, am I think it is safer to write each orbital separately, including the 2 pi bonds. Both C and N are sp hybridized because they both have two regions of electron density.
Single, double, and triple bonds are three types of covalent bonds mainly involving nonmetals. Atoms form these bonds as a way of obtaining the most stable electron configuration, according to the octet rule.
Valency Of All Elements. As with ethene, these side-to-side overlaps are above and below the plane of the molecule. The entire molecule is planar. Pi Bonds are generally weaker than sigma bonds, owing to the significantly lower degree of overlapping. C and N are triple bonded and each atom has one lone pair. Sigma and Pi Bonds The hybridization model helps explain molecules with double or triple bonds see figure below. Quick links. Triple bonds are comprised of one sigma bond and two pi bonds. So we need a more complex picture that works for all these electrons. What Are Oxides. Then the next two are pi bonds!

0 thoughts on “How many pi bonds in a triple bond”