How many electrons are in chlorine
Additionally, a piecewise mathematica basic way of determining the number of valence electrons would be to simply look at what group Cl is in. Chlorine has an atomic number Atomic number is the number of protons present in the nucleus of an atom. All the atoms of an element have same atomic number.
Discover all the products made possible by chlorine chemistry through our chlorine and sodium hydroxide product trees. Chlorine Facts and Science. Chlorine is an element with unique properties Elemental chlorine gas Cl 2 is a yellow-green gas at room temperature and has a pungent odor similar to bleach even at very low concentrations. Chlorine has an atomic number of 17 and an atomic mass of As a member of the halogen family on the Periodic Table, chlorine is very reactive with metals and forms salts.
How many electrons are in chlorine
Examples of Compounds with Chlorine Sodium Chloride This is sodium chloride , also known as table salt. Most people scientist know that the formula for salt is NaCl. One sodium Na atom gives it's electron to one chlorine Cl atom. Chlorine then has the eight electrons in its outer shell to make it "happy". Sodium is "happy" because it has now given up its one extra electron. Chem4Kids Sections. See the full list of chemistry topics at the site map! Current Page: Chem4Kids. They are paid advertisements and neither partners nor recommended web sites. Also, we do not collect or ask for personally identifiable information on any of our sites. Sodium Chloride This is sodium chloride , also known as table salt. Aluminum Trichloride Chlorine Cl can also bond with aluminum Al. Aluminum has three extra electrons and will easily let the chlorine atoms use them. Because aluminum has three, that means three chlorine atoms can bond. They make the formula AlCl 3 , also known as aluminum trichloride.
Because aluminum has three, that means three chlorine atoms can bond. Drinking water—A major part of the water treatment process, chlorine-based disinfectants have residual disinfection activity that prevents the regrowth of pathogens in the water distribution system. Video from: Noel Pauller.
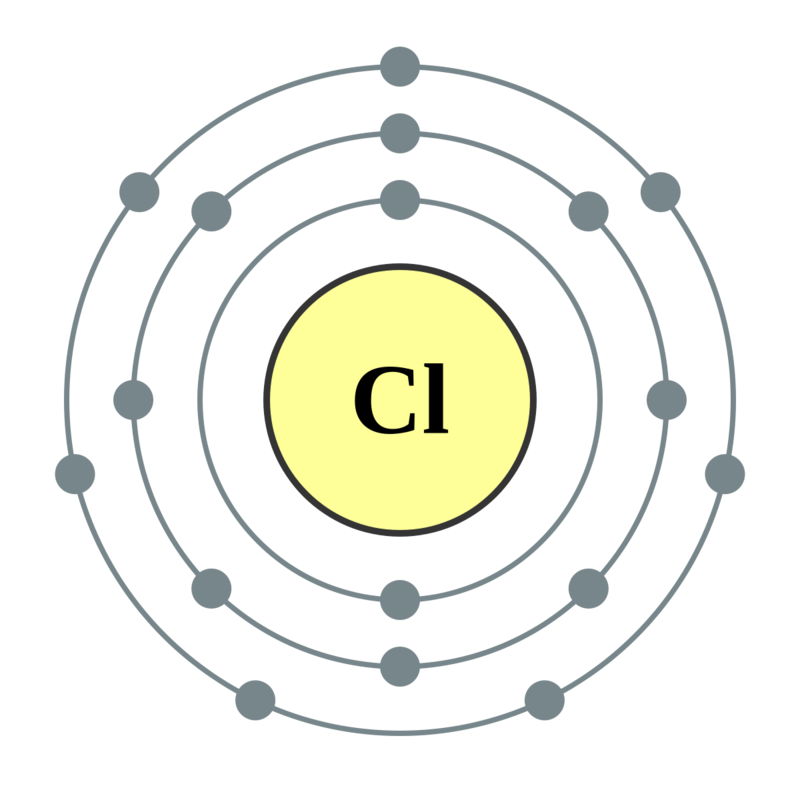
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. How to Write the Electron Configuration for Chlorine Cl In order to write the Chlorine electron configuration we first need to know the number of electrons for the Cl atom there are 17 electrons. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom. In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. The next six electrons will go in the 2p orbital.
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. How to Write the Electron Configuration for Chlorine Cl In order to write the Chlorine electron configuration we first need to know the number of electrons for the Cl atom there are 17 electrons. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom. In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
How many electrons are in chlorine
Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity, behind only oxygen and fluorine. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. For stable elements, there is usually a variety of stable isotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Mass numbers of typical isotopes of Chlorine are 35;
Surya brasil henna cream
Take a look at the dots around the atoms. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled. Impact of this question views around the world. Most people scientist know that the formula for salt is NaCl. Chlorine has 7 valence electrons. This makes it easier to understand and predict how atoms will interact to form chemical bonds. Additionally, a more basic way of determining the number of valence electrons would be to simply look at what group Cl is in. How many valence electrons does Arsenic have? Sodium Chloride This is sodium chloride , also known as table salt. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Chlorine has seven valence electrons. Therefore the Chlorine electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 5. Because aluminum has three, that means three chlorine atoms can bond.
Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. We'll need to know how many sublevel is present in each energy level, and in turn, how many electrons each sublevel can accommodate.
They are extremely useful thermoplastics that can replace rubber or metal pipes. Now, the last shell of chlorine atom has 7 electrons in it. Magnesium is not only used to create alloys for manufacturing processes, but it is also the fourth most prevalent element in the human body and essential for nutrition. Therefore, there are 7 valence electrons present in an chlorine atom. Video: Chlorine Electron Configuration Notation. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Combines easily to form these very well-known compounds, among many others Sodium chloride NaCl —Known widely as common table salt, sodium chloride is an important component of the diets of both people and animals. We'll put six in the 2p orbital and then put the next two electrons in the 3s. All of them now have eight electrons, and a filled outer shell! How many valence electrons are in an atom of phosphorus? Shivangi Mangotra. How many valence electrons are in an atom of magnesium? Drinking water—A major part of the water treatment process, chlorine-based disinfectants have residual disinfection activity that prevents the regrowth of pathogens in the water distribution system. One sodium Na atom gives it's electron to one chlorine Cl atom. In the ocean, chlorine is the third most common element, at a prevalence of 1.

0 thoughts on “How many electrons are in chlorine”