H2 i2 2hi
A: Equilibrium constant of any reaction can be obtained by dividing the h2 i2 2hi of…. Q: A sample of CaCO, s is introduced into a sealed container of volume 0. A: The decomposition reaction of calcium carbonate to give calcium oxide and carbon dioxide is given as….
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Steven Berry Modified over 5 years ago. When 4. What is the value of K?
H2 i2 2hi
Sidney W. Benson , R. This provides a reasonable explanation for some of the anomalies observed in this system and indicates that further kinetic study of the system is desirable. Sign In or Create an Account. Search Dropdown Menu. Advanced Search Citation Search. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 23, Issue 1. Previous Article Next Article. Article Navigation. Research Article January 01
Problem 48E: At a particular temperature, 8. What does the magnitude of this
Hey there! We receieved your request. Please choose valid name. Please Enter valid email. Please Enter valid Mobile. Select Grade 6th 7th 8th 9th 10th 11th 12th 12th Pass Please choose the valid grade. Register Now.
The equilibrium concentration of I2 is 0. In other words, the reaction consumed "2. Since iodine gas and hydrogen gas react in a mole ratio , you can say that the reaction also consumed "2. Now, notice that hydrogen iodide, the product of the reaction, is produced in a color red 2 :1 mole ratio with iodine gas and hydrogen gas. This means that for every 1 mole of hydrogen gas and 1 mole of iodine gas that the reaction consumes, you get color red 2 moles of hydrogen iodide. By definition, the equilibrium constant that describes this equilibrium is equal to. The answer is rounded to three sig figs. Notice that the concentrations of the two reactants decrease significantly as the reaction proceeds.
H2 i2 2hi
Hey there! We receieved your request. Please choose valid name.
Keno results
We will notify you when Our expert answers your question. Hey there! Equilibrium Calculations Chapter You do not currently have access to this content. Srinivasan R. IBr Initial: 0. Oxtoby, H. Benzaldehyde, a flavoring agent, is obtained by the dehydrogenation of benzyl alcohol. Is it true that the value of K depends on the amounts of reactants A: According to Le Chatelier's principles if some factors like temperature, pressure, molar….
.
Definition a state of balance; no net change in a dynamic. Studying in Grade 6th to 12th? In one experiment 1. Isopropyl alcohol is the main ingredient in rubbing alcohol. Problem 47E: At a particular temperature, Equilibrium Chemistry— Introduction. When a reactant The total solution volume is 1. Kp When the reactants and products are gases, we can determine the equilibrium constant in terms of partial pressures. Benson ; Sidney W. Calculate the minimum pressure…. Recommended textbooks for you. Chapter Questions Section: Chapter Questions. Do Now 1.

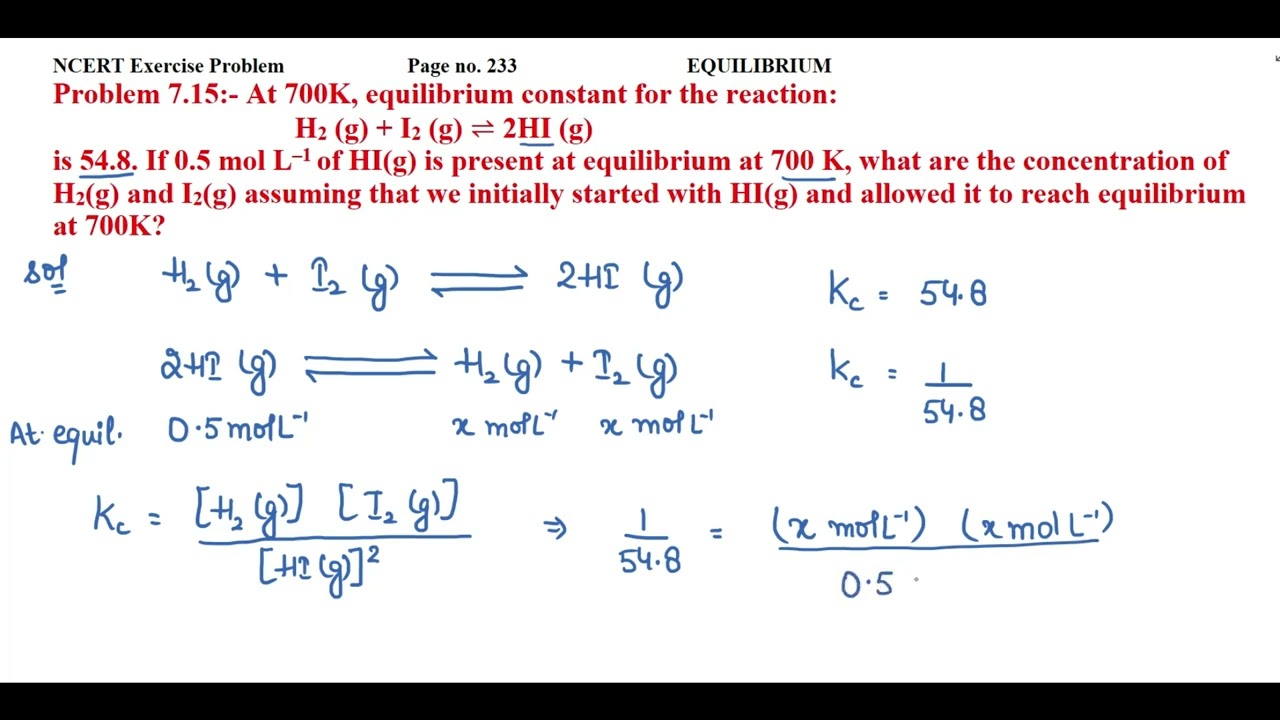
0 thoughts on “H2 i2 2hi”