Equivalent mass of copper
Distinguish among the different physical states of matter. Define the following a equivalent mass of an acid b equivalent mass of a base c equivlent mass of an oxidising agent d equivalent mass of a reducing agent. Choose the incorrect statement.
Ask your doubts live everyday Join our live doubt clearing session conducted by our experts. Give tests to analyze your progress and evaluate where you stand in terms of your JEE preparation. The equivalent mass of chlorine is The equivalent mass of copper chloride is Hence, formula of copper chloride is. None of these.
Equivalent mass of copper
Statement II : Equivalent weight of any metal is the gm quantity of metal which is combined with 8 gm of oxygen in the formation of metal oxide. Assertion : Equivalent weight of C u in C u O is The equivalent weight of iron in ferric chloride is At. If Zn has an equivalent weight of Heating mixture of C u 2 O and C u 2 S will give. A mixture of NH 3 g and N 2 H 4 g is placed in a sealed containe Chemical absorbes can be used to remove exhaled CO 2 of space travell Copper forms two oxides. For the same amount of copper, twice as much The mass of one litre sample of ozonised oxygen at NTP was found to be A sample of gaseous hydrocarbon occupying 1. Determine the formula of ammonia from the following data: i Volume Igniting MnO 2 in air converts it quantitatively to Mn 3 O 4.
Calculate the oxidation number of underlined elements in the following In the following reaction, 2 mol MnO 2 reacts with 4 mol of HCl to form An organic compound contains
.
Forgot password? New user? Sign up. Existing user? Log in. Already have an account? Log in here.
Equivalent mass of copper
In chemistry , equivalent weight also known as gram equivalent [1] or equivalent mass is the mass of one equivalent , that is the mass of a given substance which will combine with or displace a fixed quantity of another substance. The equivalent weight of an element is the mass which combines with or displaces 1. These values correspond to the atomic weight divided by the usual valence ; [2] for oxygen gas as example that is Equivalent weight has the units of mass, unlike atomic weight , which is now used as a synonym for relative atomic mass and is dimensionless. Equivalent weights were originally determined by experiment, but insofar as they are still used are now derived from molar masses. The equivalent weight of a compound can also be calculated by dividing the molecular mass by the number of positive or negative electrical charges that result from the dissolution of the compound. The first equivalent weights were published for acids and bases by Carl Friedrich Wenzel in John Dalton 's first table of atomic weights suggested a reference point, at least for the elements : taking the equivalent weight of hydrogen to be one unit of mass. One of the greatest problems was the reaction of hydrogen with oxygen to produce water.
Mr bumpy
Chemical absorbes can be used to remove exhaled CO 2 of space travell One atom of an element weighs Its atomic mass is easy View solution. Calculate the amount of water produced by the combustion of 32 g of me Video Solution. Was this answer helpful? Define limiting reagent. A mixture contains NaCl and unknown chloride MCl. Distinguish among the different physical states of matter. Was this answer helpful? Copper crystal has a face -centred cubic lattice structure. Relative molarcular mass of sulphuric acid is. A sample of gaseous hydrocarbon occupying 1. Arsenic forms two oxides, one of which contains How much volume of chlorine is required to prepare In the following reaction, 2 mol MnO 2 reacts with 4 mol of HCl to form
In the last few articles, we have studied the hydrogen displacement method, oxide formation method, reduction method, chloride formation method, and double displacement method to determine the equivalent mass of metal. The equivalent mass of a substance is the number of parts by mass of the substance which combines with or displaces or contains 1. If the equivalent mass is expressed in grams then it is called gram equivalent mass GEM.
Copper crystallizes in a cubic lattice structure. How many moles of hydrogen is required to produce 20 moles of ammonia? Video Solution. Calculate the density of copper. Chemical absorbes can be used to remove exhaled CO 2 of space travell The molecular formula of the compound is easy View solution. Justify the following reaction is a redox reaction. Mention any 4 redox reaction that takes place in our daily life. Determine the formula of ammonia from the following data: i Volume Hence, formula of copper chloride is. Was this answer helpful? A precipitate of AgCl and AgBr weighs 0. A steady current of

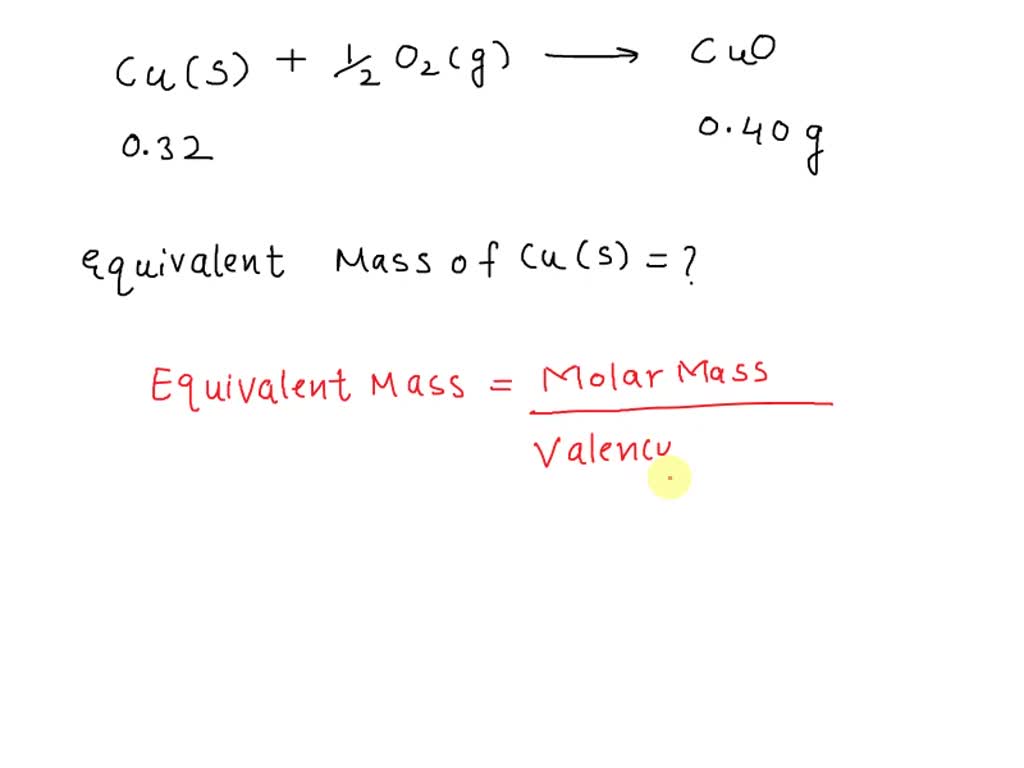
It is rather valuable information
In my opinion you are mistaken. Let's discuss it. Write to me in PM, we will talk.