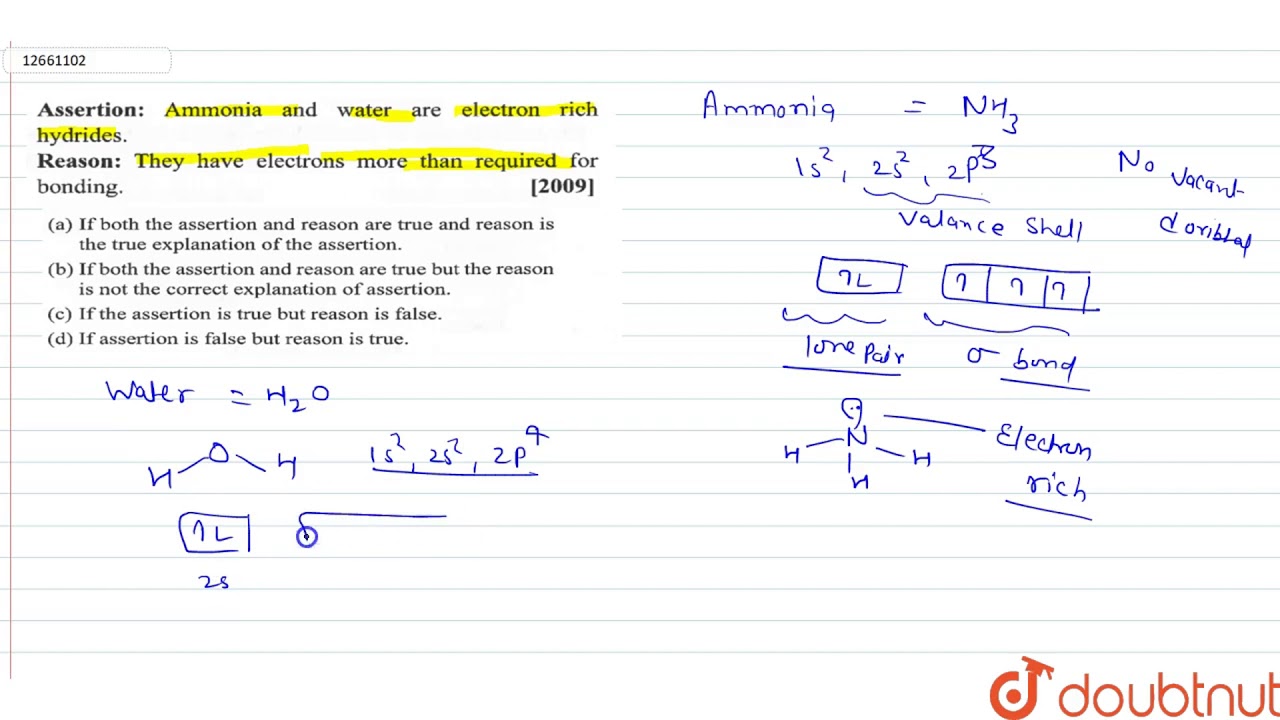
Electron rich hydrides
N H 3 is electron rich hydride.
Define the following terms: a electron-deficient b electron - precise c electron-rich compound. Justify your answer with an example? Example: CH 4 , SiH 4 , etc. What do you understand by i electron-deficient, ii electron-precise, and iii electron-rich compounds of hydrogen? Provide justification with suitable examples.
Electron rich hydrides
Hydrides - Hydrides are the binary compound of hydrogen with other elements. Let 'X" be any element, then -. Ex - MgH 2 is the hydride of Magnesium. Based upon the number of electrons and bond type covalent or molecular hydrides are classified into -. So, match each hydrides accordingly we get, a - iv , b - i , c - ii , d - iii. Last updated on Jan 2, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams.
CISF Tradesman. Hence, the correct answer is option 2. Bank of India PO.
.
As described in Section 8. In the case of neutral atomic hydrogen, this orbital is occupied by one electron. Consequently, the chemistry of hydrogen is distinguished by stable bonding arrangements in which the 1 s orbital is "filled" by one of the following:. Common bonding arrangements for hydrogen. The E-H and EE bonds in the bridging hydride represent sharing of two or more electrons among the three atoms. At room temperature and pressure, elemental hydrogen exists in the form of dihydrogen, H 2. Dihydrogen is a colorless, odorless gas that finds wide industrial application.
Electron rich hydrides
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. A new class of catalysts based on ternary ruthenium complex hydrides are developed for low-temperature ammonia synthesis. They support a non-dissociative reaction path for dinitrogen reduction, in which lithium or barium cations stabilize the N x H y intermediates and the electron- and H-rich [RuH 6 ] 4— anionic centres facilitate an energetically balanced multi-step reaction for ammonia synthesis. This is a preview of subscription content, access via your institution. Springer Nature Ltd.
No mans sky derelict freighter
Odisha Livestock Inspector. Northern Coalfields Limited. SSC Stenographer. SSC Selection Post. Rajasthan High Court Clerk. RBI Security Guard. More General Science Questions Q1. Indian Bank SO. Gujarat Police. Madras High Court Office Assistant. JEE Advanced.
Hydride , in simple terms, is said to be the anion of hydrogen. It is a chemical compound where the hydrogen atoms exhibit nucleophilic, basic or reducing properties. Compounds of hydrogen with less electronegative elements are known as hydrides.
MP Civil Judge. CWC Junior Superintendent. TN TRB. Maharashtra Arogya Vibhag Group C. Punjab Pre-Primary Teacher. HP Forest Guard. Maharashtra Zilla Parishad Rigman. BSF SI. Gujarat Administrative Service. NWDA Exam. KVS Stenographer. CG Vyapam SI. MBA Entrance Exam. Kerala Beat Forest Officer.

I join. I agree with told all above.
I am assured, that you have misled.