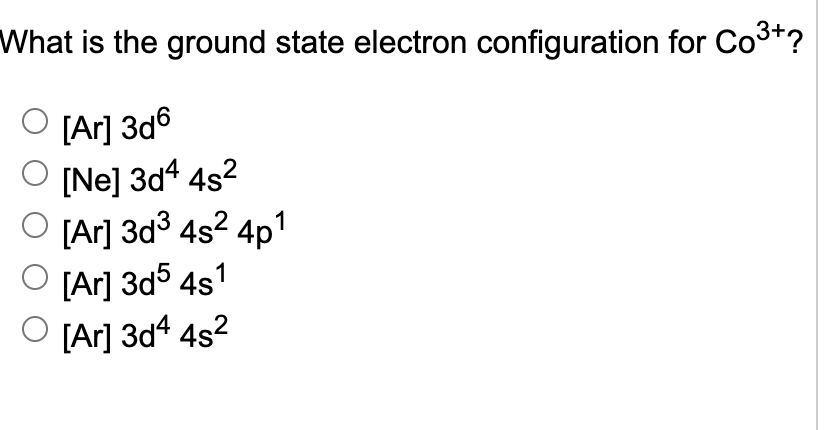
Electron configuration of co+3
Cobalt is also in Group 9, so it must have 9 valence electrons.
In this article, I have discussed in detail how to easily write the complete electron configuration of cobalt. The total number of electrons in cobalt is twenty-seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in cobalt in specific rules in different orbits and orbitals is called the electron configuration of cobalt. The electron configuration of cobalt is [ Ar ] 3d 7 4s 2 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways.
Electron configuration of co+3
.
Nobelium is a classified actinide element. Note: The abbreviated electron configuration of cobalt is [Ar] 3d 7 4s 2. When writing an electron configuration, you have to write serially.
.
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration , starting with finding the atomic number by looking at the list of orbitals and understanding the notation. But wait — you can avoid all this if you use our tool! Save time and effort by simply selecting the name of the element, and you'll get the complete electron configuration , along with the atomic number and mass of all known elements. What's more, this tool also works as a valence electron calculator and gives the valence electrons of all the elements of the periodic table. Are you ready to excel in your science course? Read on to find out what an electron configuration is , what valence electrons are , and how to find valence electrons of any element. Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons occupy.
Electron configuration of co+3
Cobalt is the 27th element of the periodic table so its atomic number is The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, a cobalt atom has twenty-seven protons and twenty-seven electrons. The number of neutrons in an atom can be determined by the difference between the atomic mass and the number of protons. The difference between the mass number of the cobalt atom and the number of protons is thirty-two. Therefore, a cobalt atom has thirty-two neutrons. The number of neutrons depends on the isotope of the element. The cobalt atom has one stable isotope. This article discussed in detail how to easily find the number of protons, neutrons, and electrons in a cobalt atom.
Definition of antonym
Electron configuration through orbit Bohr principle Electron configuration through orbital Aufbau principle Cobalt Co atom electron configuration Bohr model. What is the electron configuration for a sodium ion? What is the electron configuration of cobalt? The atomic number of cobalt is What are some examples of electron configurations? How do electron configurations affect properties and trends of a compound? The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital. Therefore, the next two electrons enter the 2s orbital. The sub-energy levels are known as s, p, d, and f. Try the Electron Configuration Calculator and get instant results for any element.
Cobalt is also in Group 9, so it must have 9 valence electrons. When a transition metal forms an ion, the s electrons are removed before the d electrons.
In this article, I have discussed in detail how to easily…. The electron configuration of all the elements can be done through the orbital diagram. What are some examples of electron configurations? The serial number of the orbit]. The complete idea of the orbit is given there. The main proponents of this principle are scientists Niels Bohr and Pauli. That is, the number of electrons in cobalt is twenty-seven. How do electron configurations affect properties and trends of a compound? Each orbital can have a maximum of two electrons. In this article, I have discussed in detail how to easily write the complete electron configuration of cobalt. Terbium is a classified lanthanide element. The sub-energy levels are known as s, p, d, and f. Atomic number, atomic weight and charge of cobalt ion. Cobalt is also in Group 9, so it must have 9 valence electrons. In this article, I have discussed in detail how to easily write the complete electron configuration of germanium….

0 thoughts on “Electron configuration of co+3”