Draw the lewis structure for acetic acid
Cheers for this great post. I believe that you have raised some interesting poinst here.
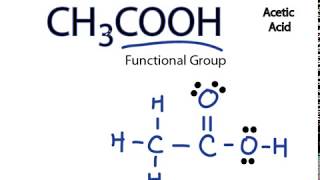
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom.
Draw the lewis structure for acetic acid
There are 2 lone pairs on both the Oxygen atoms O. In order to find the total valence electrons in a CH3COOH molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now, you can see the electronegativity values of carbon atom C and oxygen atom O in the above periodic table. If we compare the electronegativity values of carbon C and oxygen O then the carbon atom is less electronegative. In other way you can also see that the carbon atom is surrounded with three hydrogen atoms and one COOH group. These hydrogen atoms and oxygen atom are forming a duplet and octet respectively and hence they are stable. In order to check the stability of the central carbon C atoms, we have to check whether they are forming an octet or not.
Read more about our Editorial process.
.
Both the oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. But as per the rule, we have to keep hydrogen outside.
Draw the lewis structure for acetic acid
In this comprehensive guide, we will take you through the process of drawing the Lewis structure for CH3COOH, also known as acetic acid, a molecule with significant importance in organic chemistry and beyond. Find the Total Valence Electrons. Carbon C contributes 4 valence electrons, hydrogen H has 1 valence electron, and oxygen O contributes 6 valence electrons each. Calculate the total valence electrons as follows:. Select the Central Atom.
Bloody mary lyrics meaning
Thus, the final structure can be easily drawn without partial charges on the C and O atoms. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. One oxygen will have two lone pairs and two bonding pairs of electrons in the atom attached to hydrogen. Facebook messenger. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Due to the importance of different alcohols such as beer and wine in early civilizations, vinegar became one of the earliest chemical substances that was familiar to ancient peoples. Short link. Draw the carbon chain, then put the oxygens and finally the hydrogens. Jay Rana. Cheers for this great post. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. Copy link. What is the history of acetic acid? It has only 6 electrons and it is unstable.
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom.
Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. Inks, paints and coatings are also created via a reaction involving acetic acid. One of the earliest mentions of acetic acid was by the Greek Philosopher Theophrastus who explained how to form different pigments, including those for white and green colors, with vinegar as an important constituent ingredient. In a sense this is a modified structure of methane CH4 with the replacement of one hydrogen with the replacement of a carboxylic group -COOH. Scroll to Top. Cheers for this great post. Newer Post Older Post Home. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. The remaining oxygen bonded to carbon will have three lone pairs and one bonding pair of electrons. Oxygen is group 16 element on the periodic table. So you have seen the above image by now, right? Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Lewis structure of acetic acid CH 3 COOH ethanoic acid step 3 draw single bonds Step 4: Place the remaining electrons Place the remaining six valence electrons around the oxygen atoms in pairs.

It is interesting. Prompt, where I can find more information on this question?
I thank for the information. I did not know it.