Draw the electron dot structure for ethyne
Method for calculating electron dot structure:.
Past Year - 3 Mark Questions. Last updated at May 29, by Teachoo. Molecular formula of Ethane is C 2 H 6. Molecular formula of Ethene is C 2 H 4. Molecular formula of Ethyne is C 2 H 2. Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class.
Draw the electron dot structure for ethyne
.
The least electronegative atom among the given molecule will be chosen as the central atom of the molecule or ion. Draw the electron dot structure of ethyne and also draw its structural formula? Method for calculating electron dot structure: We can determine the electron dot structure of any given compound by the following steps: First, the total number of valence electrons present in the molecule has to be calculated by adding the individual valencies of each atom, which can be given as: V.
.
So far we have focused primarily on two simple types of molecular compounds: homodiatomic molecules such as H 2 , F 2 , N 2 and O 2 [22] , and binary compounds such as water. But we saw in the previous section that hydrogen and oxygen can also form another compound, namely hydrogen peroxide, H 2 O 2. Both H 2 O and H 2 O 2 are binary compounds of these elements, but the ratio of the elements is different in them: the H:O ratio in water is , but in H 2 O 2. How many other binary compounds between these two elements exist? For example, the molecule H 2 O 5 Figure seems like a reasonable candidate for a stable molecule in that the octets of all of the oxygen are satisfied and all the oxygen atoms have 2 bonds, as expected because the valence of oxygen is two and each hydrogen atom has its preferred dyad and meets its normal valence of one. But this particular structure has never been observed and therefore serves as an important example: just because you can draw what seems to be a good electron dot structure for a molecule does not mean it will exist.
Draw the electron dot structure for ethyne
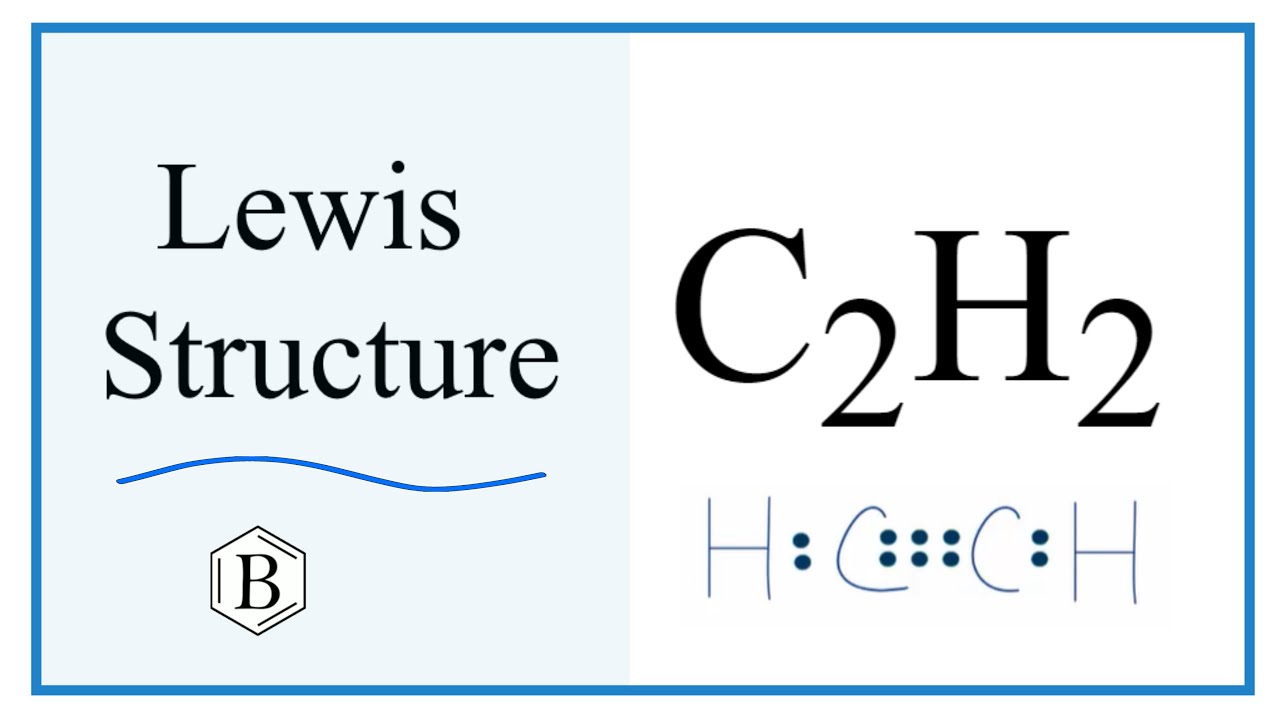
Menu Categories. Draw the electron dot structure of ethyne and also draw its structural formula. Tutorialspoint Simply Easy Learning. Updated on: Oct Related Articles Write the molecular formula of ethene and draw its electron dot structure. Draw the possible isomers of the compound with molecular formula C3H6O and also give their electron dot structures. Structural formula of ethyne is" What will be the formula and electron dot structure of cyclopentane? Write the reaction involved in this process. What type of bonds are present in water molecule? Draw the electron-dot structure of water H2O.
832 area code
Now, the lone pairs of electrons will be assigned to each atom belonging to the molecule, which can satisfy their octet configuration. It can be defined as the nature of the bond and position of atoms of the molecule which are connected within the molecule. Your browser does not support the audio element. Standard X Chemistry. Write the molecular formula of the following compounds and draw their electron-dot structures: i Ethane ii Ethene iii Ethyne Answer 1. Here the central atom is carbon, whereas Hydrogen is chosen as the side atom. We can determine the electron dot structure of any given compound by the following steps:. Hence, the electron dot structure of C 2 H 2 can be shown as: Hence, the electron dot structure of ethyne C 2 H 2 has been drawn above. Question 30 Drawthe electron dot structure of ethyne and also draw its structural formula. Covalent Bonding in H2, N2 and O2. It can be defined as the nature of the bond and position of atoms of the molecule which are connected within the molecule. E represents the total number of valence electrons and B. Draw the electron dot structure of ethyne and also draw its structural formula? Molecular formula of Ethane is C 2 H 6.
Method for calculating electron dot structure:. We can determine the electron dot structure of any given compound by the following steps:. E represents the total number of valence electrons and B.
The least electronegative atom among the given molecule will be chosen as the central atom of the molecule or ion. Hi, it looks like you're using AdBlock :. To help Teachoo create more content, and view the ad-free version of Teachooo Open in App. Method for calculating electron dot structure: We can determine the electron dot structure of any given compound by the following steps: First, the total number of valence electrons present in the molecule has to be calculated by adding the individual valencies of each atom, which can be given as: V. Now, the lone pairs of electrons will be assigned to each atom belonging to the molecule, which can satisfy their octet configuration. Displaying ads are our only source of revenue. Hence, the electron dot structure of ethyne C 2 H 2 has been drawn above. Last updated at May 29, by Teachoo. Here the central atom is carbon, whereas Hydrogen is chosen as the side atom. Trending search 3. Past Year - 3 Mark Questions. Join Teachoo Black. The electron dot structure of Ethyne:. It can be defined as the nature of the bond and position of atoms of the molecule which are connected within the molecule.

You have hit the mark. Thought excellent, it agree with you.
Anything!
I consider, that you are not right. I suggest it to discuss.