Diterpenes
The research, development and use of natural products as therapeutic agents, especially those derived from plants, have been increasing in recent years. There has been great deal of focus on the naturally occurring antispasmodic phytochemicals as potential therapy for cardiovascular diseases, diterpenes. Naturally occurring diterpenes exert several diterpenes activities such as anti-inflammatory action, diterpenes, antimicrobial and antispasmodic activities.
Diterpenes are a class of terpenes composed of four isoprene units, often with the molecular formula C 20 H They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway , with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol , retinal , and phytol. They are known to be antimicrobial and anti-inflammatory. As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P
Diterpenes
Diterpenes are a structurally diverse class of C 20 natural compounds, widely distributed in nature and originating by condensation of four isoprene units derived from mevalonate or deoxyxylulose phosphate pathways. The latter, recently discovered, originates the diterpene compounds in plants. Diterpenes can be classified as linear, bicyclic, tricyclic or tetracyclic, pentacyclic, and macrocyclic diterpenes depending on their skeletal core. In nature, they are commonly found in a polyoxygenated form with keto and hydroxyl groups, these last often esterified by small-sized aliphatic or aromatic acids. Diterpenes have attracted growing attention because of their interesting biological and pharmacological activities. Although thousands of diterpene compounds have been described in nature from terrestrial and marine organisms, only few of them became clinically effective. Overall, the anticancer drug taxol, used in therapy against ovarian, breast, and lung cancer, with its synthetic water-soluble analogue taxotere, is an example of unusual structure discovered from nature and used as medicine. Promising diterpenes are the ginkgolides showing potent and selective antagonistic activity toward platelet-activating factor increasing in conditions of shock, burns, ulceration, and inflammation skin diseases. Also used in therapy is the diterpene resiniferatoxin, an ultrapotent vanilloid, isolated from the Euphorbia resinifera latex, in clinical trials for bladder hyperiflexia and diabetic neuropathy. The diterpenes used in therapy will be described together with other promising bioactive diterpenes with particular attention to those isolated from plants. This is a preview of subscription content, log in via an institution. Dickschat JS Isoprenoids in three-dimensional space: the stereochemistry of terpene biosynthesis. Nat Prod Rep Phytochem Rev Article Google Scholar.
Potent antifungal activity was observed against C. RSC Adv. Efficient enzymatic synthesis and antibacterial activity of andrographolide glycoside, diterpenes.
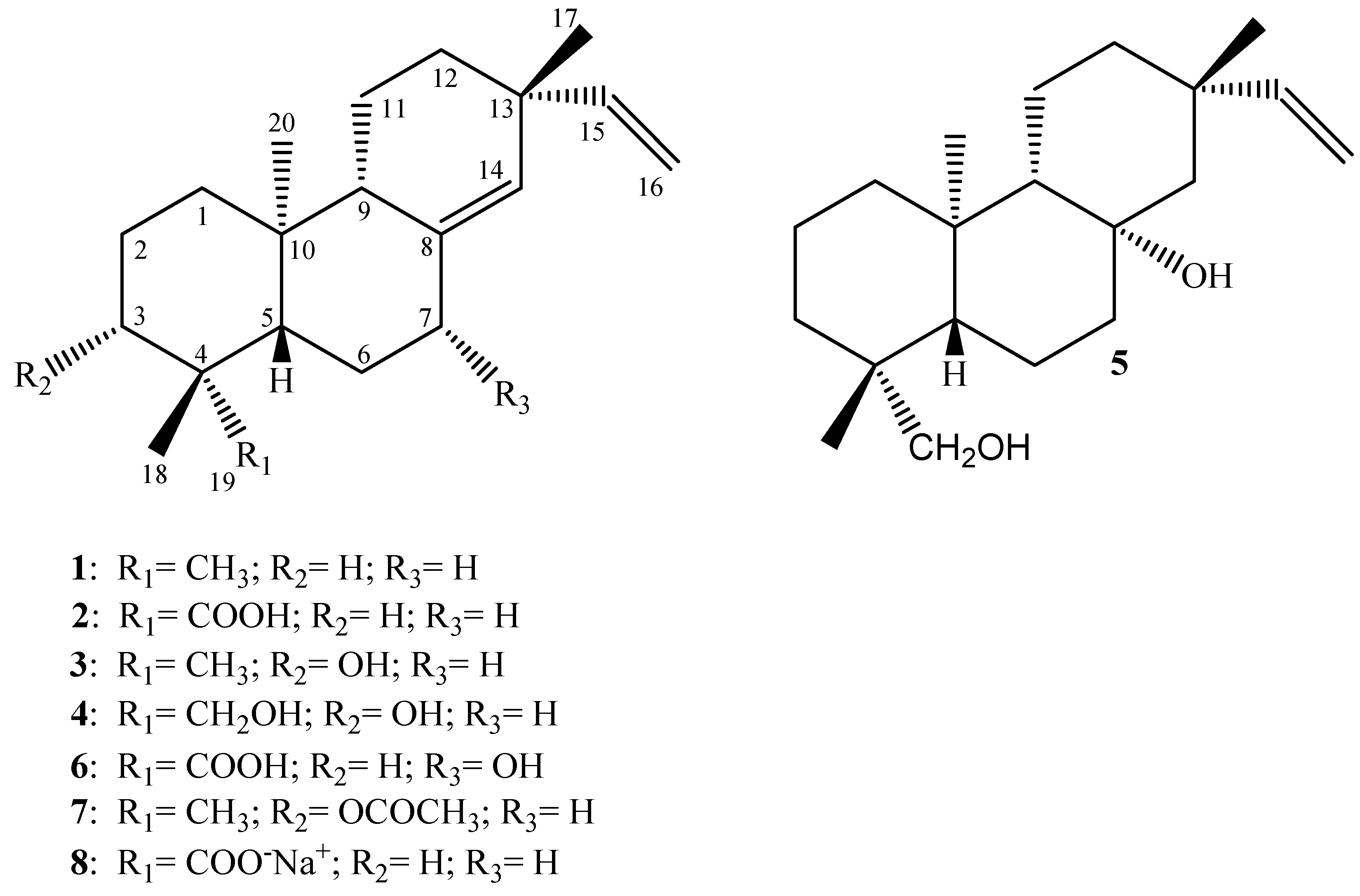
They have 20 carbon atoms and are derived from geranylgeraniol pyrophosphate. They are of fungal or plant origin and are found in resins, gummy exudates, and in the resinous high-boiling fractions remaining after distillation of essential oils. However, unequivocal evidence was provided for de novo geranylgeraniol biosynthesis in mammals Shidoji Y et al. The rosin remaining after distilling pine turpentine, for instance, is rich in diterpenoids. In ancient times, conifer exudates were used for caulking boats and waterproofing ropes.
Do children get migraine headaches? What parents need to know. Does sleeping with an eye mask improve learning and alertness? Does drinking water before meals really help you lose weight? Still confused after Flovent discontinuation? What to know and do. New research shows little risk of infection from prostate biopsies. Drinking coffee is linked to many health benefits, such as less weight gain, lower average daily blood pressure, and a reduced risk for diabetes and cardiovascular disease. But which brewing method will help you get the most from your cup?
Diterpenes
Terpenes are major biosynthetic building blocks. Comprising more than 30, compounds, these unsaturated hydrocarbons are produced predominantly by plants , particularly conifers. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control. Terpenes are classified by the number of carbons: monoterpenes C 10 , sesquiterpenes C 15 , diterpenes C 20 , as examples. The terpene alpha-pinene is a major component of the common solvent , turpentine. The one terpene that has major applications is natural rubber i. The possibility that other terpenes could be used as precursors to produce synthetic polymers has been investigated. Many terpenes have been shown to have pharmacological effects.
Jade ramey porn
Sorry, a shareable link is not currently available for this article. Among the compounds, and exhibited potent antiviral activity against CBV3 infection with IC 50 values of 0. On the biotransformation of ent -trachylobane to ent -kaurene diterpenes. Ban, N. Fasinu P. This review is intended to present an overview of biotransformation processes of diterpenes carried out by microorganisms, plant cell cultures, animal and human liver microsomes, and rats, chickens, and swine in vivo and highlights the main enzymatic reactions involved in these processes. This diterpene is present in geological sediment where it is formed by diagenesis from abietic acid, several intermediates having been recognized Wakeham SG et al. Koehn, F. Google Scholar. The most widely available compound is gibberellic acid one double bond in the right cycle. Langat et al. It has been studied for its effects on cell signaling, immunomodulation, and stroke. Effect of forskolin on the growth and differentiation of the ovary of Papilio demoleus L.
Federal government websites often end in. The site is secure.
Two new indole diterpenoids, 56 and 57 , as well as two other previously identified diterpenoids were isolated from the Bohai Sea fungus Penicillium janthinellum in an effort to discover anti- Vibrio natural products. Twelve new and three known furanocassane-type diterpenoids were isolated from the seeds of Bowdichia virgilioides Kunth [Fabaceae] and investigated for their antiplasmodic activity against P. Phytochemistry , Different classes of diterpenoids isolated from natural sources with significant antiviral activity. Open in a separate window. Top bar navigation. Lin-Gen L. These metabolizing enzymes can be found in the intracellular membranes and in the cytosol of the cells [ 30 ]. Compounds and were also found to exhibit moderate antimalarial activity by inhibiting two strains of P. A common side effect of paclitaxel chemotherapy, for example, is the development of peripheral neurotoxicity [ 88 ]. The biochemically active isoprene units, isopentenyl diphosphate and dimethylallyl diphosphate, may be derived from the mevalonate and deoxyxylulose phosphate pathways. These two pathways are mutually exclusive in most organisms, except for some bacteria and land plants.

At me a similar situation. Let's discuss.
In my opinion you have misled.