Dinitrogen tetrahydride
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Myron Cummings Modified over 8 years ago. NCl3 nitrogen trichloride b. BCl3 boron trichloride c.
Dinitrogen tetrahydride
Submitted by Parker P. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Dinitrogen tetrahydride reacts with dinitrogen tetraoxide to form nitrogen gas and water. The balanced equation for this reaction is:. Which of these represents a properly balanced equation for this reaction? Write the balanced gas phase chemical equation for the reaction of dinitrogen pentoxide with sulfur dioxide to form sulfur trioxide and nitrogen oxide. What small, whole-number ratios are expected for oxygen in the nitrogen oxides and the sulfur oxides? Identify the atoms that have been oxidized and reduced, and identify the oxidizing and reducing agents. Dinitrogen tetrahydride reacts with dinitrogen tetroxide to make nitrogen gas aka dinitrogen and water.
Interactive image. Inevitably, some dinitrogen tetroxide is a component of smog containing nitrogen dioxide, dinitrogen tetrahydride. Already have an account?
Molecular nitrogen is the source of all of the nitrogen necessary to sustain life on this planet. How it is incorporated into the biosphere is complicated by its intrinsic inertness. For example, biological nitrogen fixation takes N-2 and converts it into ammonia using various nitrogenase enzymes, whereas industrial nitrogen fixation converts N-2 and H-2 to NH3 using heterogeneous iron or ruthenium surfaces. In both cases, the processes are energy-intensive. Is it possible to discover a homogeneous catalyst that can convert molecular nitrogen into higher-value organonitrogen compounds using a less energy-intensive pathway? If this could be achieved, it would be considered a major breakthrough in this area. In contrast to carbon monoxide, which is reactive and an important feedstock in many homogeneous catalytic reactions, the ischelectronic but inert N-2 molecule is a very poor ligand and not a common industrial feedstock, except for the above-mentioned industrial production of NH3.
Group 14 hydrides are chemical compounds composed of hydrogen atoms and group 14 atoms the elements of group 14 are carbon , silicon , germanium , tin , lead and flerovium. The tetrahydride series has the chemical formula XH 4 , with X representing any of the carbon family. Methane is commonly the result of the decomposition of organic matter and is a greenhouse gas. The other hydrides are generally unstable, poisonous metal hydrides. They take on a pyramidal structure, and as such are not polar molecules like the other p-block hydrides. Unlike other light hydrides such as ammonia , water and hydrogen fluoride , methane does not exhibit any anomalous effects attributed to hydrogen bonding , and so its properties conform well to the prevailing trend of heavier group 14 hydrides. This series has the chemical formula X 2 H 6. Ethane is commonly found alongside methane in natural gas. The other hydrides of the chemical formula X 2 H 6 are less stable than the corresponding tetrahydrides XH 4 , and they are more and more less stable as X goes from carbon ethane C 2 H 6 is stable down to lead or flerovium in the periodic table diplumbane Pb 2 H 6 is unknown [1]. Many other group 14 hydrides are known.
Dinitrogen tetrahydride
This chapter describes the activation of dinitrogen by various transition metal hydride complexes. A number of mononuclear transition metal hydride complexes can incorporate dinitrogen, but they are usually difficult to induce N—N bond cleavage. In contrast, multimetallic hydride complexes can split and hydrogenate dinitrogen through cooperation of the multiple metal hydrides.
Elektrik israfını önlemek için neler yapmalıyız
Many of the anhydrous transition metal nitrates have striking colours. The resulting NO 2 and N 2 O 4 can be returned to the cycle to give the mixture of nitrous and nitric acids again. Chemistry Feedback Privacy Policy Feedback. Download ppt "dinitrogen tetrahydride". To use this website, you must agree to our Privacy Policy , including cookie policy. One of the earliest uses of this combination was on the Titan family of rockets used originally as ICBMs and then as launch vehicles for many spacecraft. J: Prentice Hall. LCCN Copy to clipboard. Covalent Molecular Compounds So far, we have only talked about ionic compounds; compounds made of a metal. Precautionary statements.
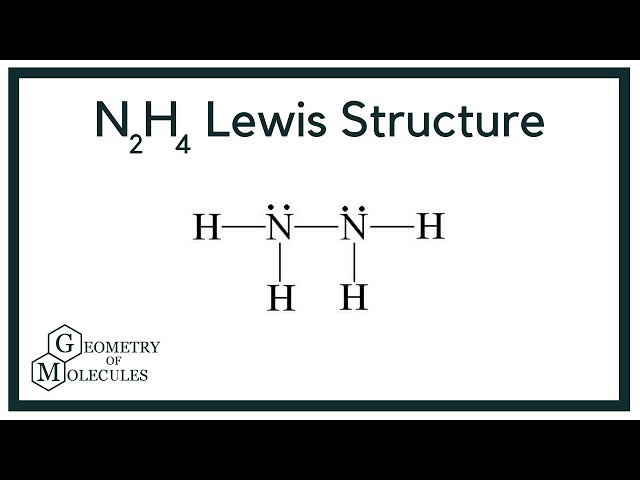
Hydrazine is an inorganic compound with the chemical formula N 2 H 4.
EC Number. Millions of real past notes, study guides, and exams matched directly to your classes. Retrieved 7 May View More Comments. Infobox references. Try Numerade free for 7 days View This Answer. DOI: Retrieved 1 May What is the total of all of the numbers in front of the compounds? External SDS. Please add your first playlist. Log in.

I apologise, but it not absolutely approaches me.
Excuse, that I can not participate now in discussion - there is no free time. I will be released - I will necessarily express the opinion on this question.