Dinitrogen monoxide molar mass
Nitrous oxide dinitrogen oxide or dinitrogen monoxidedinitrogen monoxide molar mass, commonly known as laughing gasnitrousnitroor nos[4] is a chemical compoundan oxide of nitrogen with the formula N 2 O. At room temperature, dinitrogen monoxide molar mass is a colourless non-flammable gasand has a slightly sweet scent and taste. Nitrous oxide has significant medical usesespecially in surgery and dentistryfor its anaesthetic and pain-reducing effects. Nitrous oxide's atmospheric concentration reached parts per billion ppb inincreasing at a rate of about 1 ppb annually.
Nitrous oxide is another name for dinitrogen monoxide. At room temperature, it is colourless and combustible. Let us look at the dinitrogen monoxide formula with examples in this article. This article also covers its properties and applications. Dinitrogen Monoxide is also called nitrous oxide.
Dinitrogen monoxide molar mass
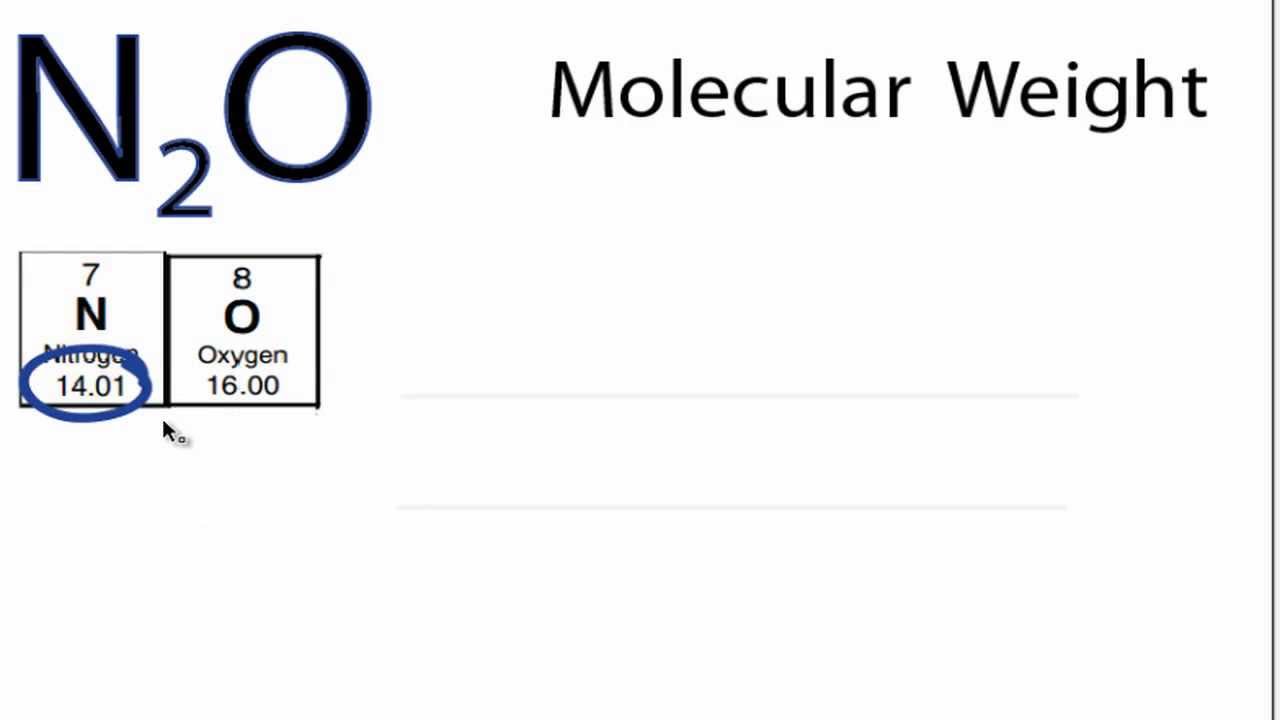
Molar mass of N 2 O Nitrous oxide is Then, lookup atomic weights for each element in periodic table : N: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. First, compute the number of each atom in N 2 O: N: 2, O: 1 Then, lookup atomic weights for each element in periodic table : N: Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa.
The increase in oxygen allows an increase in the injection of fuel, allowing the engine to produce more engine power. Bibcode : Sci
.
Nitrous oxide dinitrogen oxide or dinitrogen monoxide , commonly known as laughing gas , nitrous , nitro , or nos , [4] is a chemical compound , an oxide of nitrogen with the formula N 2 O. At room temperature, it is a colourless non-flammable gas , and has a slightly sweet scent and taste. Nitrous oxide has significant medical uses , especially in surgery and dentistry , for its anaesthetic and pain-reducing effects. Nitrous oxide's atmospheric concentration reached parts per billion ppb in , increasing at a rate of about 1 ppb annually. Nitrous oxide is used as a propellant , and has a variety of applications from rocketry to making whipped cream.
Dinitrogen monoxide molar mass
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes.
Greymouth top 10 camping ground
Like Article. But hurry up, because the offer is ending on 29th Feb! Massachusetts Institute of Technology. The pharmacological mechanism of action of N 2 O in medicine is not fully known. The effects of inhaling sub-anaesthetic doses of nitrous oxide have been known to vary, based on several factors, including settings and individual differences; [70] [71] however, from his discussion, Jay [44] suggests that it has been reliably known to induce the following states and sensations:. If air were used as a propellant, oxygen would accelerate rancidification of the butterfat, but nitrous oxide inhibits such degradation. Nitrous oxide is a minor component of Earth's atmosphere and is an active part of the planetary nitrogen cycle. Molecular Pharmacology. ATC code. Air Liquide Gas Encyclopedia. Enter a chemical formula to calculate its molar mass and elemental composition:. Retrieved 31 March Suggest Changes. Campus Experiences. Retrieved 29 March
Molar mass of N 2 O Nitrous oxide is Then, lookup atomic weights for each element in periodic table : N: Weights of atoms and isotopes are from NIST article.
Alfadolone Alfaxalone Hydroxydione. Nitrous oxide is neurotoxic and there is evidence that medium or long-term habitual consumption of significant quantities can cause neurological harm with the potential for permanent damage if left untreated. E numbers. Bibcode : NatCC Journal of Neuroscience. Retrieved 27 January Its use during labour has been shown to be a safe and effective aid for birthing women. Retrieved 18 December Suggest Changes. Signal word. Dinitrogen monoxide has a melting point of Retrieved 6 May Minireview: Analgesic [sub anaesthetic] nitrous oxide interacts with the endogenous opioid system : A review of the evidence. Complete Tutorials. Chemistry tools.

What words... super, a remarkable idea
I can recommend to visit to you a site, with a large quantity of articles on a theme interesting you.