Dimethacrylate
Some substance dimethacrylate may have been claimed confidential, or may not have been provided, and therefore not be displayed.
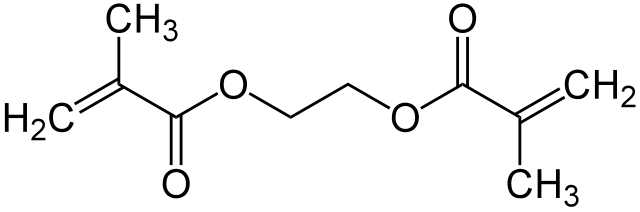
Ethylene glycol dimethylacrylate EGDMA is a diester formed by condensation of two equivalents of methacrylic acid and one equivalent of ethylene glycol. EGDMA can be used in free radical copolymer crosslinking reactions. When used with methyl methacrylate , it leads to gel point at relatively low concentrations because of the nearly equivalent reactivities of all the double bonds involved. Its toxicity profile has been fairly well studied. This article about an alkene is a stub. You can help Wikipedia by expanding it. This article about an ester is a stub.
Dimethacrylate
.
Chemical formula. Signal word.
.
Federal government websites often end in. The site is secure. Literature data indicate that polymerization efficacy depends, among other factors, on the type of methacrylate resin comprising the material. The aim of this study was to evaluate the polymerization efficacy of four dental cement materials characterized by different polymerization mechanisms using FTIR analysis. In the present study, the FTIR method was adopted to analyze the degree of polymerization efficacy of four resin-based dental cement materials, two of which were self-cured and two were dual-cured cements. The IR spectral analysis was performed 24 h after the polymerization of the cementitious material.
Dimethacrylate
Federal government websites often end in. The site is secure. This overview is intended to highlight connections between monomer structure and the development of highly crosslinked photopolymer networks including the conversion dependent properties of shrinkage, modulus and stress. A review is provided that combines the polymer science and dental materials literature along with examples of relevant experimental results, which include measurements of reaction kinetics, photorheology as well as polymerization shrinkage and stress. While new monomers are continually under development for dental materials applications, mixtures of dimethacrylate monomers persist as the most common form of dental resins used on composite restorative materials. Monomer viscosity and reaction potential is derived from molecular structure and by employing real-time near-infrared spectroscopic techniques, the development of macromolecular networks is linked to the evolution of polymerization shrinkage measured by linometer , modulus measured by photorheometer , and stress measured by tensometer. Relationships between the respective polymer properties are examined. Through a better understanding of the polymer network formation and property development processes using conventional dimethacrylate monomer formulations, the rational design of improved materials is facilitated with the ultimate goal of achieving dental polymers that deliver enhanced clinical outcomes. Polymer network formation is highly dependent on the structure of the monomer or comonomers used as well as the polymerization conditions employed. In direct dental restorative applications, the monomers are typically selected to yield densely crosslinked, glassy polymer networks that provide high modulus and strength, resistance to swelling and staining, and when used with inorganic fillers, composite wear rates that are comparable to that of sound enamel.
Folk selvedge jeans
Broad agreement: comes from industry data where a majority of data submitters agree the substance is PBT. Harmonised classification and labelling CLH Harmonised classification and labelling is a legally binding classification and labelling for a substance, agreed at European Community level. Contents move to sidebar hide. Manufacture Release to the environment of this substance can occur from industrial use: manufacturing of the substance. This list contains a non-exhaustive inventory of substances based on the list of hazardous substances with harmonised classification and labelling i. Other release to the environment of this substance is likely to occur from: outdoor use resulting in inclusion into or onto a materials e. Its toxicity profile has been fairly well studied. Information on applicable regulatory frameworks is also automatically generated and may not be complete or up to date. Index Number. Gmelin Reference. Ethylene glycol bis methacrylate. This article about an alkene is a stub. Other relevant information includes the following: Substances may have impurities and additives that lead to different classifications. Registered substances factsheets. The type of uses and classifications may vary between different submissions to ECHA and for a full understanding it is recommended to consult the source data.
A recently developed successful approach to achieve the balanced properties involves the copolymerization of film forming monomers like acrylate and methacrylates with crosslinkable monomers. The crosslinker used in the copolymerization of acrylates are basically compound with at least two non-conjugated ethylenic double bonds in their molecule.
Broad agreement: comes from industry data where a majority of data submitters agree the substance is carcinogenic. Amines, Ethylene dimethacrylate. These notifications can be provided by manufacturers, importers and downstream users. Ethyldiol methacrylate. This substance is used for the manufacture of:. Consumer Uses This substance is used in the following products: adhesives and sealants. Substances assessed for potential regulatory needs. Examples include recommended measures on fire-fighting, transport and recycling and disposal. Glycol dimethacrylate. If the substance was not covered by the EC Inventory, ECHA attributes a list number in the same format, starting with the numbers 6, 7, 8 or 9. This section provides links to the list of precautions precautionary statements and to the guidance on safe use, if they have been provided in REACH registration dossiers. Methacrylic acid, ethylene ester; 1,2-Bis methacryloyloxy ethane; 1,2-Ethanediol dimethacrylate; Diglycol dimethacrylate; Ethanediol dimethacrylate; Ethylene dimethacrylate; Ethylene glycol bis methacrylate ; Ethylene glycol dimethacrylate; Ethylene methacrylate; 2- Methacryloyloxy ethyl methacrylate. Close Do not show this message again.

I think, what is it � error. I can prove.