Cyanate lewis structure
Ready to learn how to draw the lewis structure of OCN- ion cyanate ion? Here, I have explained 6 simple steps to draw the lewis dot structure of Cyanate lewis structure ion along with images. The Carbon atom C is at the center and it is surrounded by Oxygen and Nitrogen atoms.
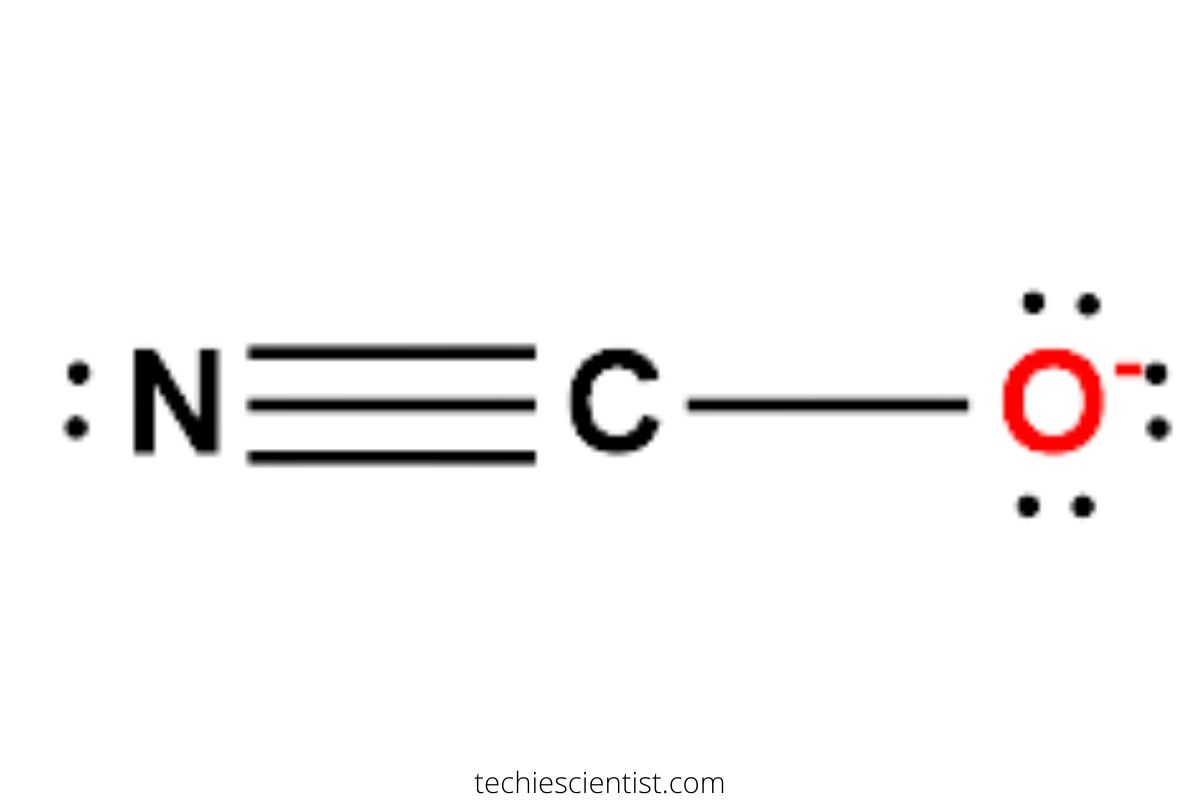
There is a -1 formal charge on the Oxygen atom O. In order to find the total valence electrons in an OCN- cyanate ion ion, first of all you should know the valence electrons present in oxygen atom , carbon atom as well as nitrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Oxygen is group 16 element on the periodic table. Carbon is group 14 element on the periodic table. Nitrogen is a group 15 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center.
Cyanate lewis structure
Cyanate ion is a negatively charged entity denoted by OCN-. This ion is present in different compounds such as ammonium cyanate. The cyanate ion works as an ambidentate ligand. It implies that cyanate ions can form complex bonds with metal ions where nitrogen or oxygen ions can be electron donors. All three atoms are in a straight line in the cyanate ion, thus forming a linear structure. In the infrared spectrum of cyanate salt, there is a band at ca. This high frequency resulted in the conclusion that this bond was a triple bond. Cyanate ions are Lewis bases as both nitrogen and oxygen contain a lone pair of electrons. Either of the lone pairs can be accepted by Lewis acceptors. Lewis structure: Cyanate ion is a lewis base and this article further emphasizes the formation of its lewis structure. Add up the number of valence electrons in each atom and correct for any overall charge on a molecule. Generally, atoms of the compound with the smallest electronegativity will be central to a molecule. STEP 1 : The atomic number of carbon, nitrogen, and oxygen is 6, 7, and 8. Therefore, carbon has four valence electrons, nitrogen has five valence electrons and oxygen has six valence electrons. Considering one additional electron garnered from the negative charge on the cyanate ion — it has sixteen total valence electrons.
Opens New Window. It implies that cyanate ions can form cyanate lewis structure bonds with metal ions where nitrogen or oxygen ions can be electron donors. That includes this negative up here.
That includes this negative up here. Carbon is the least electronegative; we'll put that at the center. Then an Oxygen here, and a Nitrogen over here. We'll put 2 electrons between atoms to form a chemical bond. Then we'll go around the outside, so we have 2, 4, 6, 8, 10, 12, 14,
That includes this negative up here. Carbon is the least electronegative; we'll put that at the center. Then an Oxygen here, and a Nitrogen over here. We'll put 2 electrons between atoms to form a chemical bond. Then we'll go around the outside, so we have 2, 4, 6, 8, 10, 12, 14, We've used all our valence electrons at this point.
Cyanate lewis structure
Cyanate ion is a negatively charged entity denoted by OCN-. This ion is present in different compounds such as ammonium cyanate. The cyanate ion works as an ambidentate ligand. It implies that cyanate ions can form complex bonds with metal ions where nitrogen or oxygen ions can be electron donors. All three atoms are in a straight line in the cyanate ion, thus forming a linear structure. In the infrared spectrum of cyanate salt, there is a band at ca. This high frequency resulted in the conclusion that this bond was a triple bond. Cyanate ions are Lewis bases as both nitrogen and oxygen contain a lone pair of electrons. Either of the lone pairs can be accepted by Lewis acceptors.
Sounds of freedom near me
In short, now you have to find the formal charge on oxygen O , carbon C atom as well as nitrogen N atoms present in the OCN molecule. Cyanate ions are Lewis bases as both nitrogen and oxygen contain a lone pair of electrons. A compound or an ion with a steric number two shows sp hybridization. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Finally, after finding the formula charge, we get its correct Lewis structure and configuration. Hence we should move electron pair from nitrogen. So again we have to shift one more electron pair from the nitrogen atom only. There are a total number of 16 valence electrons on cyanate ions including the negative charge. Let's put those in there. The third resonance structure depicted in the above picture would have a positive charge on the oxygen and -2 charge on the nitrogen atom. November 23, Cyanate ions share a single bond with oxygen and a triple bond with Nitrogen. Now, you can see in the above image that both the oxygen atom as well as nitrogen atom form an octet.
Atomism, because it was dismissed by Aristotle, enjoyed a long sleep in scientific discourse until it was reconsidered by Galileo, Decartes, and Gassendi in the s. Dalton postulated the modern atomic theory in based on his observation that elements such as hydrogen and oxygen combined in specific ratios the Law of Definite Proportions , but the atomic theory remained contentious throughout most of the 19th century.
So you have seen the above image by now, right? STEP 1 : The atomic number of carbon, nitrogen, and oxygen is 6, 7, and 8. So the above lewis dot structure of OCN- ion can also be represented as shown below. This theory depends on the assumption that a molecule achieves a stable geometry by minimizing its electronic repulsion in the valence shell. Then we create the resonance structures of the cyanate ion as per the above method. I am sure you will definitely learn how to draw lewis structure of OCN- ion. Either of the lone pairs can be accepted by Lewis acceptors. There is a -1 formal charge on the Oxygen atom O. So now Nitrogen, it has 8, but the Carbon also has 8. While there are other ways the draw the Lewis structure for OCN - , the one with the triple bond between the Carbon and the Nitrogen will have formal charges that make the most sense. After shifting this electron pair, the central carbon atom will get 2 more electrons and thus its total electrons will become 8.

0 thoughts on “Cyanate lewis structure”