Cuno3 naoh
Direct link to this balanced equation:. A chemical equation represents a chemical reaction.
Post by » Tue Oct 05, am. Laurence Lavelle Skip to content. Quick links. Email Link. My question is how to get the net ionic equation for the reaction question a.
Cuno3 naoh
I keep rereading what's in my textbook over and over again, but I'm just lost! Could someone please help and explain! Many thanks! You're dealing with a double replacement reaction in which two soluble ionic compounds react to form an insoluble solid that precipitates out of the aqueous solution. Now, notice that you need 2 moles of sodium hydroxide for every 1 mole of copper II nitrate that takes part in the reaction. To get the complete ionic equation , rewrite the soluble ionic compounds as cations and anions. Now, in order to get the net ionic equation , you must eliminate spectator ions , i. Stefan V. Mar 22, Explanation: You're dealing with a double replacement reaction in which two soluble ionic compounds react to form an insoluble solid that precipitates out of the aqueous solution.
Unit converters. This method separates the reaction into two half-reactions — one for oxidation and one for reduction. Get rid of cuno3 naoh ions, these are ions that appear the same including same stoichiometric coefficient on both sides of the equation.
.
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom.
Cuno3 naoh
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction.
Water pressure pump bunnings
CuOH is insoluble. Related questions Question fee Stefan V. This method uses algebraic equations to find the correct coefficients. Balancing with algebraic method This method uses algebraic equations to find the correct coefficients. Next, identify the solubility of each compound. Not exactly sure how to balance this equation without the Na part. There are 2 H atoms on the left and 2 H atom on the right. I keep rereading what's in my textbook over and over again, but I'm just lost! Now, write the complete ionic equation break up all soluble compounds into ions and write their charges! Why are chemical reactions important? Best for: Equations that are more complex and not easily balanced by inspection.
.
Balancing with inspection or trial and error method This is the most straightforward method. CuOH is insoluble. Question e2ea6. Previous Next: balancing chemical equations. CuOH is the only compound of the four that will not dissociate. You're dealing with a double replacement reaction in which two soluble ionic compounds react to form an insoluble solid that precipitates out of the aqueous solution. Impact of this question views around the world. Gas laws. Best for: complex redox reactions, especially in acidic or basic solutions. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. No is not balanced: 6 atoms in reagents and 3 atoms in products. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation.

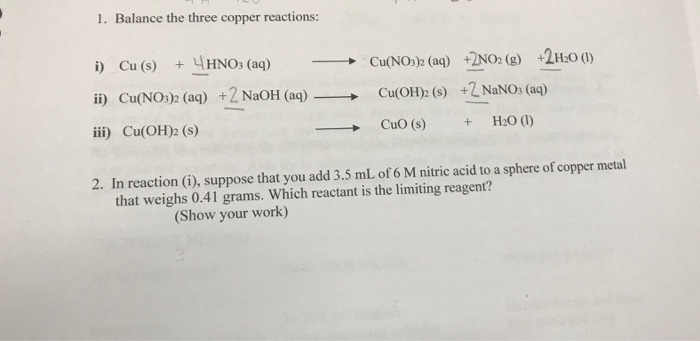
0 thoughts on “Cuno3 naoh”