Convert grams to moles
In chemistry, convert grams to moles, the Mole is used as a fundamental unit that helps us understand the world at the atomic and molecular level. But what exactly is a Mole, and why is it so important in chemistry? A mole is a unit of measure used to indicate a specific amount of substance.
The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles. Depending on the input data it can serve either as grams to moles calculator or as moles to grams calculator. It also displays the molar mass of the chemical substance and details of its calculation just for reference. You can find more information and formulas about grams to moles conversion as well as about moles to grams conversion below the calculator. Note: Always use the upper case for the first character in the element name and the lower case for the second character as in the periodic table. Compare: Co - cobalt and CO - carbon monoxide. You need to divide the mass of the substance by the molar mass of the substance:.
Convert grams to moles
Last Updated: August 21, Fact Checked. This article was co-authored by Bess Ruff, MA. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. There are 8 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed , times. Moles are a standard unit of measurement in chemistry that take into account the different elements in a chemical compound. This conversion can help give you a clearer picture of the number of molecules you're working with rather than dealing with weight, which can change between molecules. Although the conversion is simple, there are a number of important steps that need to be followed. Using this method, you can learn how to convert grams into moles. To convert grams to moles, start by multiplying the number of atoms by the atomic weight for each element in the compound. Then, add all of your answers together to find the molar mass of the compound. Finally, divide the number of grams of the compound by the molar mass of the compound to find the number of moles.
As you can see, the most difficult task here is finding out the molar mass of the substance. Understand audiences through statistics or combinations of data from different sources.
This worked example problem shows how to convert the number of grams of a molecule to the number of moles of the molecule. This type of conversion problem mainly arises when you are given or must measure the mass of a sample in grams and then need to work a ratio or balanced equation problem that requires moles. Determine the number of moles of CO 2 in grams of CO 2. First, look up the atomic masses for carbon and oxygen from the periodic table. The atomic mass of C is The formula mass of CO 2 is:.
Discover the ease of converting grams to moles with our intuitive calculator. Designed to simplify your chemical calculations, this tool sparks curiosity and enhances understanding. Our Grams to Moles Calculator is a vital tool for chemistry enthusiasts and professionals. It accurately transforms the mass of a substance in grams to the equivalent number of moles, considering the substance's molecular weight. Grasp the essence of the Grams to Moles Calculator's formula and its significance in the field of chemistry. This understanding is crucial for accurate molecular weight computations.
Convert grams to moles
How heavy is 1. How many moles in Calculating the mass of a sample from the number of moles it contains is quite simple. We use the molar mass mass of one mole of the substance to convert between mass and moles. Can you see how the units cancel to give you the answer you want? All you needed to know was that you had 1. Thus, multiplying 1. A liter of air contains 9. What is the mass of Ar in a liter of air? The molar amount of Ar is provided and must be used to derive the corresponding mass in grams.
100 pound sterling to usd
This type of conversion problem mainly arises when you are given or must measure the mass of a sample in grams and then need to work a ratio or balanced equation problem that requires moles. Rated this article:. With this grams to moles calculator, you can swiftly find how to calculate grams to moles for any substance. You can find more information and formulas about grams to moles conversion as well as about moles to grams conversion below the calculator. Use limited data to select content. Edit this Article. Wishlist 0. You Might Also Like. Avogadro's Number Example Chemistry Problem. The atomic mass is 1. How to convert grams to moles: an example Take a look at the example of how to calculate moles from grams. Do the calculation and use the calculator on this page to check your calculation. Then, multiply it by the number of moles to get an answer in grams:. Divide the mass by the molar mass to find the number of moles in your sample. Learn why people trust wikiHow.
Last Updated: August 21, Fact Checked. This article was co-authored by Bess Ruff, MA. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
Note: Always use the upper case for the first character in the element name and the lower case for the second character as in the periodic table. These choices will be signaled to our partners and will not affect browsing data. Thus, one mole of H 2 SO 4 weighs Imagine you have 6 liters of pure water:. About This Article. The molecular mass of a substance is calculated as the number of atoms of each element multiplied by the atomic weight of that element. Determine the number of atoms that each element contributes to the compound. Thanks Helpful 15 Not Helpful 8. Read on to learn the grams to moles formula, try solving a problem of how to convert grams to moles yourself, and forget having any issues converting g to mol in the future! Not Helpful 16 Helpful The molar mass of KClO3 is To convert this to the Mole unit, we divide grams by

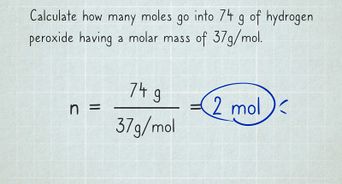
I apologise, but, in my opinion, you are not right. I am assured. I can defend the position. Write to me in PM, we will discuss.
It is a pity, that now I can not express - there is no free time. But I will be released - I will necessarily write that I think on this question.