Condensed electron configuration
Electron Config Lite Yukod Software. Dla wszystkich info. Konfiguracja elektronowa Lite to darmowa wersja naszego WE Pro aplikacji.
Numeracja egzemplarza: 21 , Bazy online. Bazy CM UJ. Katalog Bibliotek UJ. Zasoby online artykuły, rozdziały i więcej. Połączone katalogi.
Condensed electron configuration
Two-dimensional 2D monolayer materials are interesting systems due to an existence of optically non-active dark excitonic states. In this work, we formulate a theoretical model of an excitonic Auger process which can occur together with the trap-assisted recombination in such 2D structures. The interactions of intravalley excitons bright and spin-dark ones and intervalley excitons momentum-dark ones with deep states located Full text available to download. New intermetallic compounds Tb2Pd1. The crystallographic structure and magnetic, electronic transport, and thermal properties are reported. The ac magnetic susceptibility of a single crystal sample of the compound Y9Co7 has been measured in applied dc fields ranging from 0—6. A broad maximum has been observed in the zero field susceptibility measurements from 2. Full text to download in external service. Simulations with three different semi-local and dispersion-corrected X-ray diffraction, specific heat, magnetic susceptibility and inelastic x-ray scattering measurements on the transurarium oxypnictides NpFeAsO and NpFeAsO0.
Report problem zamknij okno ×.
Link to the lesson. Nagranie dostępne na portalu epodreczniki. Methane, whose molecules are composed of one carbon atom and hydrogen atoms, is the simplest hydrocarbon. The place of carbon in the periodic table indicates that it has four valence electrons. The carbon atom, combined with four hydrogen atoms, forms a compound with the formula CH 4 , i. What do we already know about it?
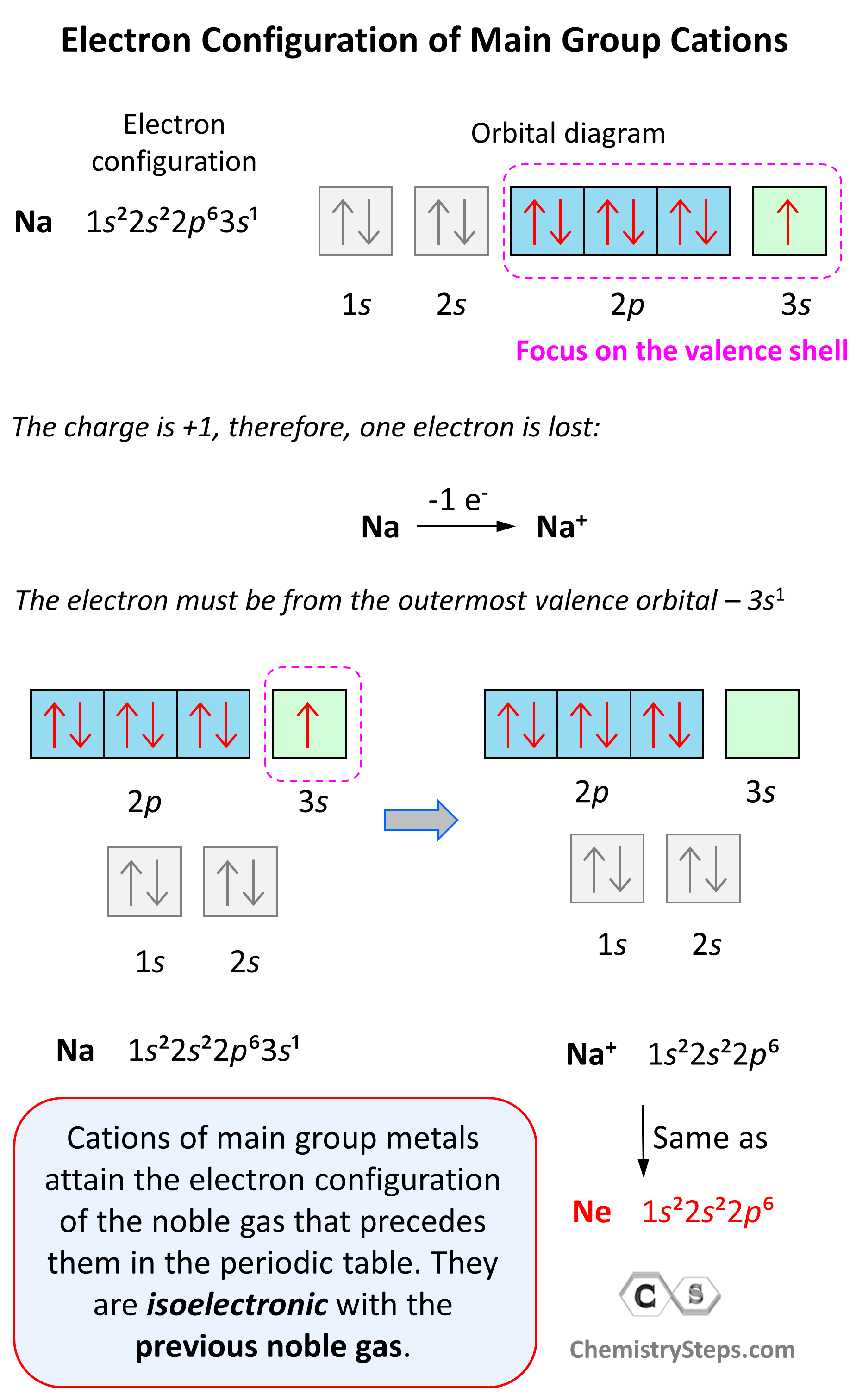
Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. This allows us to determine which orbitals are occupied by electrons in each atom. The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information Figure 1 :.
Condensed electron configuration
Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. This allows us to determine which orbitals are occupied by electrons in each atom. The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom.
Arty market coruña
Safarik T. Lazarowska M. As you already know, alkanes are saturated hydrocarbons whose molecules have only single bonds between carbon atoms. Strychalska-Nowak T. Source: GroMar Sp. Publication P. Wykonano pomiary podatności magnetycznej, oporu elektrycznego i ciepła właściwego dla VAl Disciplines Field of Science :. Quasielastic scattering. Cross-disciplinary physics: materials science. Na portalu Ludzie Nauki swoje dane z bazy Nauka Polska zobaczysz w ciągu najbliższych miesięcy. ISSN: Has funding. All bonds between carbon atom and hydrogen atom are the same the angles between bonds are °28'. Cai S.
Notice that protons go in the nucleus of the atom and electrons are drawn on orbits surrounding the nucleus. Image from Wikimedia commons.
Knowing this formula, you can easily write down the formula for any alkane: for example, an alkane with 5 carbons in the chain will have the molecular formula C 5 H Opisana została synteza i podstawowe właściwości fizyczne monokryształów CaFe2As2. Vannette J. Lis Y. Speghini M. Dostępny pełny tekst. Saturated hydrocarbons — alkanes. Springell E. Other interactions of matter with particles and radiation. Isopentane Neopentane butane propane. Champagne F. Ronning N. Etan ethane , wzór sumaryczny: c dwa ha sześć, wzór strukturalny: dwa oxnnaczone symbolami atomy węgla oraz sześć atomów wodoru - wodoru z pojedynczymi wiązaniami. The methane molecule has the shape of a regular tetrahedron. Publication T.

In my opinion you are not right. I am assured. I suggest it to discuss.