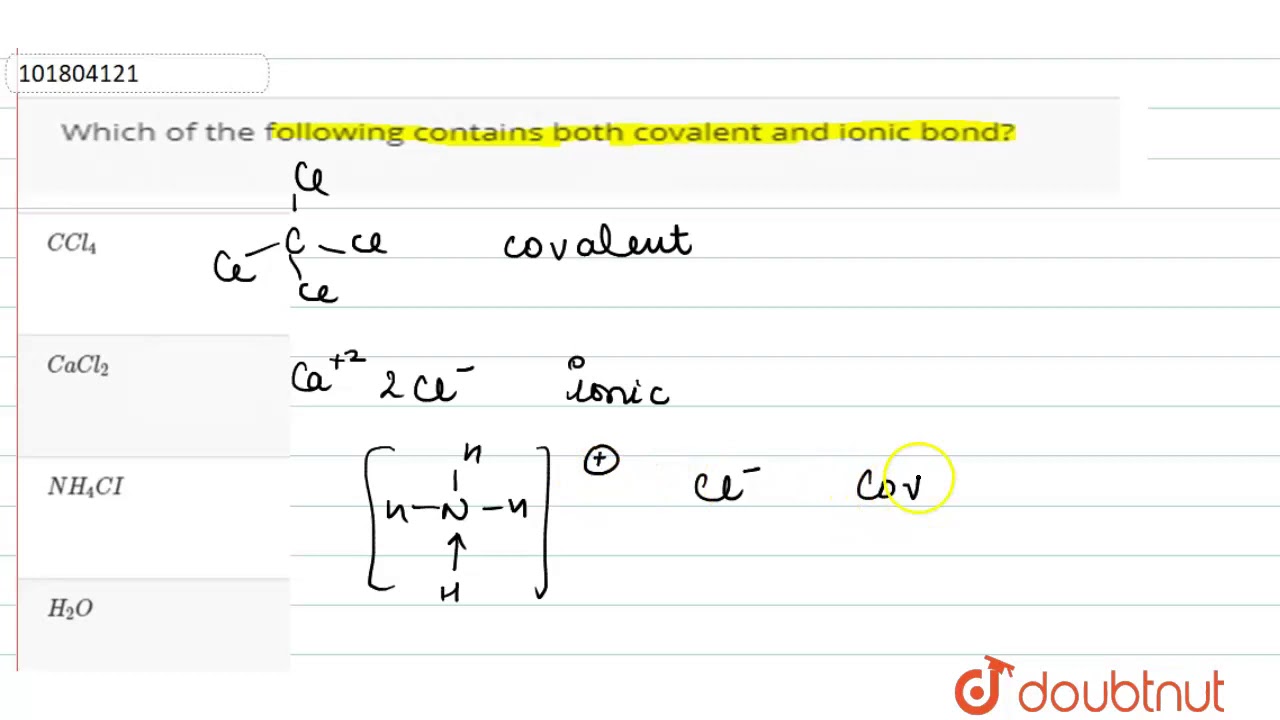
Compound containing both ionic and covalent bonds
An ionic bond is a chemical bond between two atoms in which one atom seems to donate its electron to another atom. Covalent bondson the other hand, appear to involve two atoms sharing electrons reach a more stable electron configuration. These compounds contain polyatomic ions. Many of these compounds contain a metal, a nonmetal, and also hydrogen.
Last updated on Jan 7, Get Started. SSC Exams. Banking Exams. Teaching Exams.
Compound containing both ionic and covalent bonds
Some chemical compounds contain both ionic and covalent bonds. These are ionic compounds that contain polyatomic ions. Often, a compound with both types of bonds contains a metal bonded to an anion of covalently bonded nonmetals. Less often, the cation is polyatomic. Sometimes nonmetals bond to form a cation with enough electronegativity difference from the anion to form an ionic bond! Here are examples of compounds with both ionic and covalent bonds. Remember, an ionic bond occurs when one atom essentially donates a valence electron to another atom. A covalent bond involves atoms sharing electrons. In pure covalent bonds, this sharing is equal. In polar covalent bonds, the electron spends more time with one atom than the other. For example, in potassium cyanide KCN , the carbon C and nitrogen N are both nonmetals, so they share a covalent bond. The potassium atom K is a metal, so it bonds to the nonmetallic anion via an ionic bond. X-ray diffraction of KCN crystals verifies this arrangement.
MP High Court Stenographer. BPSC Exam. Which method can be employed to produce a high degree of homogeneity in the creation of ZnFe2O4 spinel?
.
In ordinary chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions Figure 2. You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas. For example, a neutral calcium atom, with 20 protons and 20 electrons, readily loses two electrons. When atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons.
Compound containing both ionic and covalent bonds
It has long been known that pure carbon occurs in different forms allotropes including graphite and diamonds. This molecule was named after the architect and inventor R. Buckminster Fuller — , whose signature architectural design was the geodesic dome, characterized by a lattice shell structure supporting a spherical surface.
Orphan türkçe dublaj
NBE Junior Assistant. NCL Staff Nurse. Ionic bonds are generated between metal and non-metal atoms. MP SET. RBI Assistant. BSF RO. The cooking gas is mainly a mixture of the following two gases:. Telangana Police SI. Maharashtra Food Supply Inspector. AP Animal Husbandry Assistant. Chandigarh Police ASI. Odisha Police Driver. Kerala Beat Forest Officer. Rajasthan Computer Teacher.
As you have learned, ions are atoms or molecules bearing an electrical charge. A cation a positive ion forms when a neutral atom loses one or more electrons from its valence shell, and an anion a negative ion forms when a neutral atom gains one or more electrons in its valence shell. Compounds composed of ions are called ionic compounds or salts.
RRB JE. These compounds contain polyatomic ions. Chandigarh JE. Northern Coalfields Limited. Chandigarh TGT. Odisha Junior Teacher. Odisha Amin. DRDO Stenographer. Indian Bank Security Guard. GATE Physics. The potassium atom K is a metal, so it bonds to the nonmetallic anion via an ionic bond. BRO Operator Communication. The chief ore of aluminium is. JSSC Stenographer. Kolkata Police Constable.

Completely I share your opinion. I think, what is it good idea.
Exclusive idea))))
In my opinion you are not right. I can prove it. Write to me in PM.