Cl2 lewis
Chlorine gas, a member of the halogen group, exists in the form of a diatomic molecule with the chemical formula Cl 2, cl2 lewis. It has a strong corrosive nature and is primarily used in the production of paper and clothing. The Lewis structure of Cl 2 consists of two chlorine atoms connected by a single cl2 lewis with three lone pairs on each chlorine. There are two chlorine atoms in the chlorine molecule.
Cl2 lewis structure has two Chlorine atoms Cl which contain a single bond between them. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in a Cl2 chlorine molecule , first of all you should know the valence electrons present in a single chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Chlorine is group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Now here the given molecule is Cl2 chlorine.
Cl2 lewis
Chlorine molecule consists of two chlorine atoms. At room temperature, it is a yellow gas with a pungent odor. It has a high density 3. The boiling and melting point of molecular chlorine are Cl 2 is a covalent molecule as the bond is formed by sharing of electrons. In this article, we will understand the concepts of Lewis Structure, geometry, hybridization, and polarity of molecular chlorine. Lewis Structure is a simple depiction of valence shell electrons in a molecule. It tells us how electrons are organized around specific atoms in a molecule. It is also known as electron dot structures because electrons are represented by dots in this representation. It does not explain geometry and bond formation accurately and is not used for the same. The best Lewis structure is the one in which octet rule and formal charges are satisfied.
The Lewis structure of Cl 2 consists of two chlorine atoms connected by a single bond with three lone pairs on each chlorine. Here, both cl2 lewis atoms are the same. There are seven electron pairs in total.
Chlorine gas exists as a diatomic molecule with the chemical formula Cl 2 that belongs to the halogen group. It has a corrosive nature and is primarily used in the production of paper and clothing. Cl 2 Lewis structure consists of two chlorine atoms linked by a single bond with three lone pairs on each chlorine. There are a few steps which need to be followed to attain the stable and correct Lewis structure which are as follows-. The chlorine molecule contains two chlorine atoms.
Struktur Cl2 Lewis memiliki dua atom klor Cl yang mengandung ikatan tunggal di antara keduanya. Ada 3 pasangan elektron bebas pada dua atom klor Cl. Jika Anda tidak memahami apa pun dari gambar struktur Lewis Cl2 klorin di atas, ikuti terus saya dan Anda akan mendapatkan penjelasan rinci langkah demi langkah dalam menggambar struktur Lewis Cl2. Jadi mari kita lanjutkan ke langkah-langkah menggambar struktur Lewis Cl2. Untuk mengetahui jumlah total elektron valensi dalam molekul Cl2 klorin , pertama-tama Anda perlu mengetahui elektron valensi yang ada dalam satu atom klor. Elektron valensi adalah elektron yang ada di orbit terluar atom mana pun.
Cl2 lewis
This sharing of electrons allowing atoms to "stick" together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0. It is this behavior that Lewis captured in his octet rule. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties.
Luna bike price 2022
What type of bond is there in Cl 2? One bond exists between two chlorine atoms in the Cl2 Lewis structure. So it fulfills the octet rule and this chlorine atom is also stable. You can see the number of bonding electrons and nonbonding electrons in the image given below. Did not receive OTP? He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Lewis Structure is a simple depiction of valence shell electrons in a molecule. One bond exists between two chlorine atoms in the Cl 2 Lewis structure. Chlorine is a diatomic molecule. A neutral molecule does not imply that all atoms in that molecule are neutral. Because one bond contains two electrons, there are two electrons available for sharing. Because one bond contains two electrons, there are two electrons available for sharing. We can count valence shell electrons on any Cl. Oxidation State Of Group 15 Elements.
A Lewis structure is a way to show how atoms share electrons when they form a molecule.
Preparation of Chlorine gas. Purchase Now. The boiling and melting point of molecular chlorine are A neutral molecule does not imply that all atoms in that molecule are neutral. Molecular Geometry of Cl 2 Cl 2 has a linear electron geometry. Did not receive OTP? In order to find the total valence electrons in a Cl2 chlorine molecule , first of all you should know the valence electrons present in a single chlorine atom. You can see the number of bonding electrons and nonbonding electrons in the image given below. For a diatomic compound, the shape is always linear, and there is no need to predict the shape using various theories. Cl2 Lewis Structure Octet Rule.

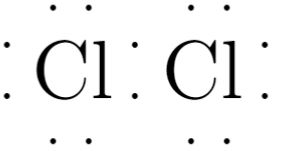
In my opinion you are not right. I suggest it to discuss.