Charge of io3
Iodates are a class of iodine -containing chemical compounds analogous to the chlorine-containing chlorates. In these compounds, an ionic bond is formed between a metal cation and the iodate anion, which consists of one atom of iodine covalently bound to three atoms of oxygen, charge of io3, and carries a formal charge of
It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. They are the salts of iodic acid. Iodate is pyramidal in structure. It participates in several redox reactions, such as the iodine clock reaction. Iodate shows no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate. Iodate is reduced by sulfite : [1]. Iodate is also obtained by reducing a periodate with a sulfide.
Charge of io3
For IO3- we have a total of 26 valence electrons. Iodine is the least electronegative. We'll put that at the center, and then we'll put the Oxygens around the outside. We have 26 valence electrons for IO We'll put 2 between atoms to form chemical bonds. We've used 6. Then we'll go around and fill the octets for the Oxygens. We have 6, 8, 10, 24; and then back to the central Iodine, So it looks like we're done: each of the atoms has 8 valence electrons, so the octets are satisfied, and we've used 26 valence electrons. However, when we look at the structure, Iodine is below period 2, row 2 on the periodic table. That means Iodine can have an expanded octet--can have more than 8 valence electrons--so we really need to look at the formal charges here to see if this is the most likely structure for IO For the Iodine--Iodine has 7 valence electrons on the periodic table.
Our formal charge for this entire structure here shows up to be -1, which makes sense. Related questions.
IO 3 — lewis structure has an Iodine atom I at the center which is surrounded by three Oxygen atoms O. There is 1 single bond and 2 double bonds between the Iodine atom I and each Oxygen atom O. There is 1 lone pair on Iodine atom I , 2 lone pairs on double bonded Oxygen atom O and 3 lone pairs on single bonded Oxygen atom O. In order to find the total valence electrons in IO3- ion, first of all you should know the valence electrons present in iodine atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Iodine is a group 17 element on the periodic table. Oxygen is group 16 element on the periodic table.
IO 3 — iodate has one iodine atom and three oxygen atoms. In IO 3 — Lewis structure, there are two double bonds and one single bond around the iodine atom, with three oxygen atoms attached to it. The oxygen atom with double bonds has two lone pairs, the oxygen atom with a single bond has three lone pairs, and the iodine atom has one lone pair. In the periodic table , iodine lies in group 17, and oxygen lies in group Hence, iodine has seven valence electrons and oxygen has six valence electrons. Now the IO 3 — has a negative -1 charge, so we have to add one more electron. Learn how to find: Oxygen valence electrons. We have a total of 26 valence electrons.
Charge of io3
In this article we are discussing about io3- lewis structure including its drawing, hybridization, shape, pairs and some FAQS. Iodate is an oxoanion of iodine. It is formed when Iodic acid losses one proton. It has the molecular weight of Usually iodates occur in nature as salts which are generally colorless. As iodine is bigger in size and has less electronegativity than O atom I act as the central atom in this compound. Iodine has 7 valance electrons out of which 3 electrons take part in sigma bonding with 3 O atoms and forms 2 pi bond with 2 O atoms. One electron pair still present on I atom which remains as lone electron pair on central I atom. O atom has 6 valance electrons out of which 1 is used in making sigma bond and 1 is used in making pi bond and remaining 4 electrons present as lone pair of electrons on O atom. Resonance means movement of electrons from one atom to another atom and the structure obtained by this process is called canonical structure.
Principality stadium seating plan
So here also you can easily say that the charge of IO 3 should be 1-, then only it will get canceled out. Wikimedia Commons. In other projects. We'll put 2 between atoms to form chemical bonds. When we draw a lewis structure, there are several guidelines to follow. Retrieved Remember that, there are total of thirteen electron pairs. So it looks like we're done: each of the atoms has 8 valence electrons, so the octets are satisfied, and we've used 26 valence electrons. This indicates that the above lewis structure of IO3 is not stable and so we have to minimize the charges to get a more stable lewis structure. We really want our formal charges to be as close to zero as possible. So now, you have to complete the octet on these oxygen atoms because oxygen requires 8 electrons to have a complete outer shell. The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. What is the chemical formula for hydrogen iodate? There is -1 charge on oxygen atom in IO 3 - lewis structure. Iodate charge of anion?
Iodate ion contains one iodine and three oxygen atoms. There is -1 charge on oxygen atom in IO 3 - lewis structure. Oxygen atoms have made bonds with center iodine atom.
Total electron pairs are determined by dividing the number total valence electrons by two. So it looks like we're done: each of the atoms has 8 valence electrons, so the octets are satisfied, and we've used 26 valence electrons. You should just try to remember that IO3 has 1- charge. Save my name, email, and website in this browser for the next time I comment. Read Edit View history. Space-filling model of the iodate anion. Additionally, iodates that do not contain toxic metals, such as potassium iodate or calcium iodate , can be used for iodine supplements or radioactivity prophylaxis treatments. Now, we know how many electrons are there in valence shells of oxygen and iodine atoms. Chemical formula for berllium iodate? Email Tweet Facebook. After determining the center atom and sketch of IO 3 - ion, we can start to mark lone pairs on atoms. P Potassium Biiodate. Download as PDF Printable version. There is 1 lone pair on Iodine atom I , 2 lone pairs on double bonded Oxygen atom O and 3 lone pairs on single bonded Oxygen atom O. Amphoteric nature of water NO 2 - lewis structure N 2 O lewis structure, resonance structures Stability of water.

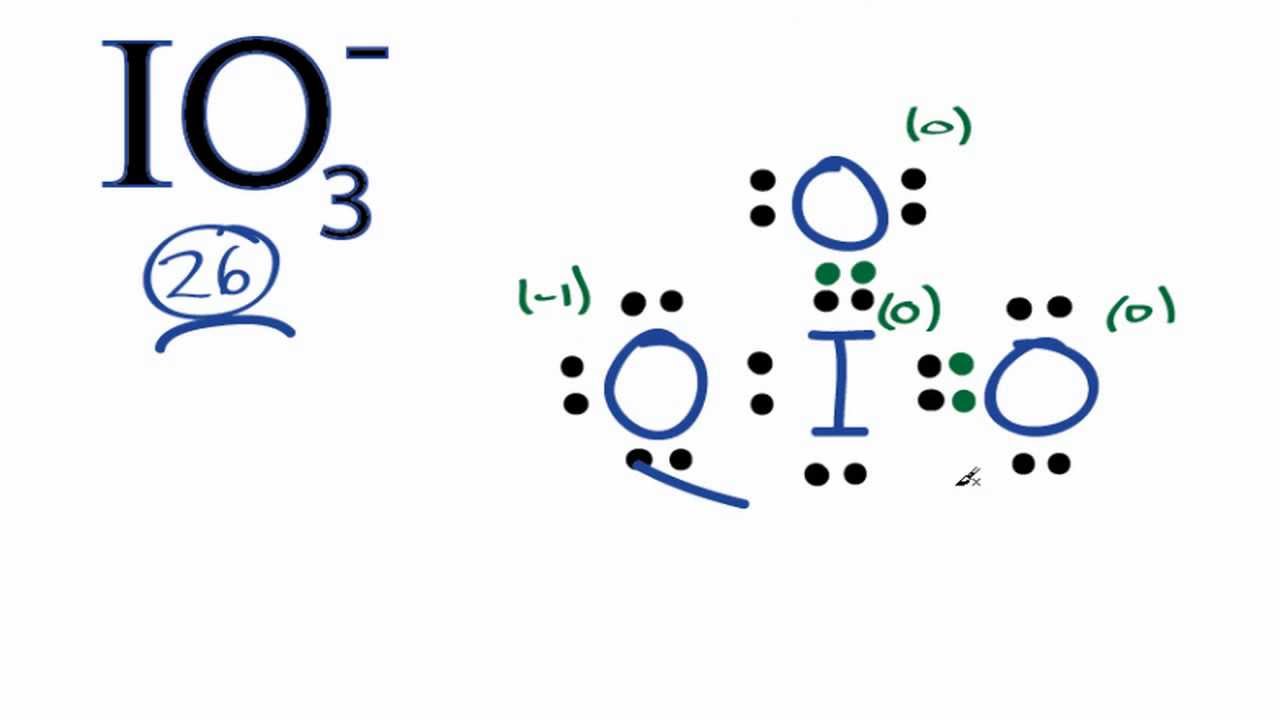
It not absolutely that is necessary for me. Who else, what can prompt?