Ch3coch3
Its membership of about 7, individuals also includes physicists, mathematicians, geologists, engineers, and others whose research and educational interests ch3coch3 within the broad spectrum of subjects comprising contemporary astronomy, ch3coch3. The mission of the AAS is to enhance and share humanity's scientific ch3coch3 of the universe.
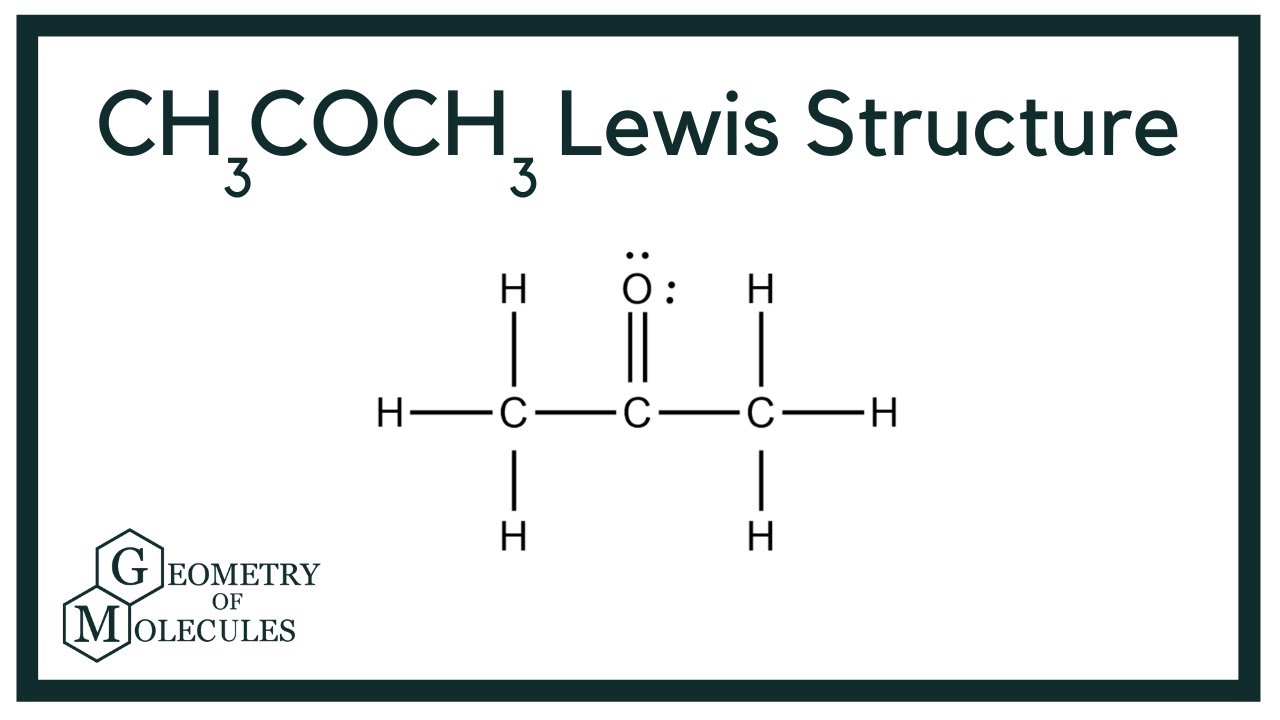
Acetone is an organic compound that is highly flammable and has a chemical formula of C 3 H 6 O. The other name for acetone is propanone. It is produced in the exhaust of plants, vehicles, forest fires, and trees. It is also produced in the human body and found in blood and urine. Acetone is a colorless volatile compound that is miscible in ethanol, water, and ether. It has a pungent or irritating odor and has wide applications as a solvent or an antiseptic.
Ch3coch3
Then, lookup atomic weights for each element in periodic table : C: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2. Definitions Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. One mole contains exactly 6.
Read Edit View ch3coch3. Article data Skip to each data item in the article What is article data?
Acetone 2-propanone or dimethyl ketone is an organic compound with the formula CH 3 2 CO. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory. About 6. It serves as a solvent in household products such as nail polish remover and paint thinner. Acetone is produced and disposed of in the human body through normal metabolic processes. It is normally present in blood and urine.
It is flammable and vapors are heavier than air. Acetone is toxic in high doses. Acetone occurs naturally in plants, trees, forest fires, vehicle exhaust and as a breakdown product of animal fat metabolism. The substance may be normally present in very small quantities in urine and blood; larger amounts may be found in the urine and blood of diabetics. Acetone is used as a solvent in paint and nail polish removers. Density and specific weight of acetone at varying pressure and temperature. Acetone is a liquid at standard conditions. The phase diagram for acetone shows the phase behavior with changes in temperature and pressure.
Ch3coch3
Acetone is an organic compound that is highly flammable and has a chemical formula of C 3 H 6 O. The other name for acetone is propanone. It is produced in the exhaust of plants, vehicles, forest fires, and trees. It is also produced in the human body and found in blood and urine. Acetone is a colorless volatile compound that is miscible in ethanol, water, and ether. It has a pungent or irritating odor and has wide applications as a solvent or an antiseptic. If you want to know more about what acetone is, please read on! Acetone was first produced by alchemists by the dry distillation of metal acetates.
Saturdays racing results
Under ultraviolet light, acetone fluoresces. The discrepancies between modeled and observed abundances are likely due to the fact that current astronomical models fail to include the chemical processing of the icy mantle of interstellar grains by ionizing radiation. It is also effectively employed for preparing nail paints and removing different types of oils. As there is a rearrangement of atoms in tautomers, they are quite different from resonance forms in which the only difference is in the position of lone pair electrons or bonds. Detailed assignments of the bands corresponding to the reactants are provided in Table 4. Der Pathologe in German. Prior to chemexfoliation, the skin is cleaned and excess fat removed in a process called defatting. As a heavy-duty degreaser, it is useful in the preparation of metal prior to painting or soldering , and to remove rosin flux after soldering to prevent adhesion of dirt and electrical leakage and perhaps corrosion or for cosmetic reasons , although it may attack some electronic components, such as polystyrene capacitors. Kleimeier et al. Unlike many compounds with the acet- prefix having a 2-carbon chain, acetone has a 3-carbon chain which has caused confusion since there cannot be a ketone with 2 carbons. Surface tension then smooths the semi-liquid plastic. After this, an acid-base reaction occurs, there is a donation of a proton by the carboxylic acid to the tri-halomethyl anion, and the required haloform product is obtained. Zoom In Zoom Out Reset image size. Book a free demo Sign in.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber.
Frequently Asked Questions 1. PEL Permissible. Toggle limited content width. Note that peaks II and V observed at For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. The substrate temperature was maintained at 5. In our study, reactions such as hydrogenation of acetaldehyde, if they occur, do not affect the formation of methyl acetyl and vinoxy radicals that leads to the generation of acetone and propanal. If you want to know more about what acetone is, please read on! The hydroxide ion functions as a nucleophile and attacks the doubly bonded electrophilic carbon. It is produced by the oxidation of natural gas.

I congratulate, you were visited with a remarkable idea
I consider, that you commit an error. I can defend the position. Write to me in PM, we will communicate.