Ch3cn lewis structure
Wiki User.
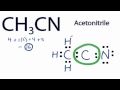
For CH3CN we have 4 valence electrons for the Carbon plus 1 for the Hydrogen we have 3 Hydrogens plus 4 for the other Carbon and then 5 for that Nitrogen, giving us a total of 16 valence electrons. Carbon's the least electronegative, so that's going to go at the center. We'll put the other Carbon here and then the Nitrogen on this side. We can tell by the way it's written, that the CH3 means we're going to have Hydrogens around this Carbon right here, and the Nitrogen will be here on the other side. So we have 3 Hydrogens around this Carbon here. We have our central Carbon, and then we have our Nitrogen over here.
Ch3cn lewis structure
There is a triple bond between the Carbon atom C and Nitrogen atom N. There is 1 lone pair on the Nitrogen atom N. In order to find the total valence electrons in a CH3CN molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as nitrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Nitrogen is a group 15 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now, you can see the electronegativity values of carbon atom C and nitrogen atom N in the above periodic table. If we compare the electronegativity values of carbon C and nitrogen N then the carbon atom is less electronegative. This indicates that these atoms are chemically bonded with each other in a CH3CN molecule.
It is an ionic compound so it would not have a Lewis dot structure. Steric No.
Acetonitrile, also known as methyl cyanide is a colorless organic liquid with an aromatic odor. It is majorly produced as a byproduct during the manufacturing of acrylonitrile. It is used in the organic synthesis of many compounds where it acts as a polar aprotic solvent. It was first produced by Jean Baptiste Dumas in It is also a potent air pollutant found in automobile and industrial exhausts.
The total number of valence electrons present in a molecule of acetonitrile will be equal to 16 because you have. Now, the two carbon atoms will be bonded together via a single bond. One of the two carbon atoms will be bonded to the nitrogen atom via a triple bond and the other will be bonded to the three hydrogen atoms via single bonds. The remaining 2 valence electrons will be added on the nitrogen atom as a lone pair. In order to find the hybridization of the two carbon atoms, you must count the regions of electron density that surround the atoms.
Ch3cn lewis structure
Note: The review of general chemistry in sections 1. Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in a molecule. While it can be helpful initially to write the individual shared electrons, this approach quickly becomes awkward. A single line is used to represent one pair of shared electrons. Line representations are only used for shared electrons. Lone pair unshared electrons are still shown as individual electrons. Double and triple bonds can also be communicated with lines as shown below. Since the lone pair electrons are often NOT shown in chemical structures, it is important to mentally add the lone pairs. In the beginning, it can be helpful to physically add the lone pair electrons. For organic chemistry, the common bonding patterns of carbon, oxygen, and nitrogen have useful applications when evaluating chemical structures and reactivity.
Digi wifi estudiantes
The molecular geometry also takes into account other factors such as the bond angles, bond strength, etc. Skip to content Acetonitrile, also known as methyl cyanide is a colorless organic liquid with an aromatic odor. Jay Rana. As there are two carbon atoms in this molecule it may have two different shapes depending on which carbon atom is chosen as the central atom. This step enables us to estimate the number of electrons that are still required by one or more atoms of the molecule to complete their octet. It is the three-dimensional positioning of its atoms that is determined by the bonding and non-bonding electrons present in that compound around the central atom. Let's move 2 more electrons here and share them with the Carbon. Previously Viewed. As discussed earlier in the case of the CH 3 CN molecule any of the carbon atoms can be chosen as central atom, therefore, two hybridization states are possible for this molecule. It is used in the organic synthesis of many compounds where it acts as a polar aprotic solvent.
The Lewis structure of CH 3 CN contains four single bonds and one triple bond, with two carbons in the center.
Carbon is group 14 element on the periodic table. Lewis, Lewis dot symbol or lewis structures are the diagrams that demonstrate the bonding between different atoms of a compound, specifying the number of bonds as well as the lone pairs of electrons. Jay Rana. It is assumed that an atom tends to form bonds in order to attain stability. November 23, The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. How much acetonitrile in 36 percent acetonitrile? No, not exactly. We'll put 2 electrons between atoms to form chemical bonds.

Excuse for that I interfere � To me this situation is familiar. I invite to discussion. Write here or in PM.